Complement in the Homeostatic and Ischemic Brain
- PMID: 26322048
- PMCID: PMC4533015
- DOI: 10.3389/fimmu.2015.00417
Complement in the Homeostatic and Ischemic Brain
Abstract
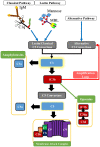
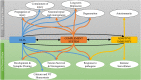
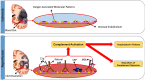
The complement system is a component of the immune system involved in both recognition and response to pathogens, and it is implicated in an increasing number of homeostatic and disease processes. It is well documented that reperfusion of ischemic tissue results in complement activation and an inflammatory response that causes post-reperfusion injury. This occurs following cerebral ischemia and reperfusion and triggers secondary damage that extends beyond the initial infarcted area, an outcome that has rationalized the use of complement inhibitors as candidate therapeutics after stroke. In the central nervous system, however, recent studies have revealed that complement also has essential roles in synaptic pruning, neurogenesis, and neuronal migration. In the context of recovery after stroke, these apparent divergent functions of complement may account for findings that the protective effect of complement inhibition in the acute phase after stroke is not always maintained in the subacute and chronic phases. The development of effective stroke therapies based on modulation of the complement system will require a detailed understanding of complement-dependent processes in both early neurodegenerative events and delayed neuro-reparatory processes. Here, we review the role of complement in normal brain physiology, the events initiating complement activation after cerebral ischemia-reperfusion injury, and the contribution of complement to both injury and recovery. We also discuss how the design of future experiments may better characterize the dual role of complement in recovery after ischemic stroke.
Keywords: brain ischemia; complement; innate immunity; neuroprotection; reperfusion injury; stroke.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

