Impact of peroxisome proliferator-activated receptor γ on angiotensin II type 1 receptor-mediated insulin sensitivity, vascular inflammation and atherogenesis in hypercholesterolemic mice
- PMID: 26322101
- PMCID: PMC4548041
- DOI: 10.5114/aoms.2015.53309
Impact of peroxisome proliferator-activated receptor γ on angiotensin II type 1 receptor-mediated insulin sensitivity, vascular inflammation and atherogenesis in hypercholesterolemic mice
Abstract
Introduction: The angiotensin II type 1 receptor (AT1R) and the peroxisome proliferator-activated receptor γ (PPARγ) have been implicated in the pathogenesis of atherosclerosis. A number of studies have reported that AT1R inhibition or genetic AT1R disruption and PPARγ activation inhibit vascular inflammation and improve glucose and lipid metabolism, underscoring a molecular interaction of AT1R and PPARγ. We here analyzed the hypothesis that vasculoprotective anti-inflammatory and metabolic effects of AT1R inhibition are mediated by PPARγ.
Material and methods: Female ApoE(-/-)/AT1R(-/-) mice were fedwith a high-fat and cholesterol-rich diet and received continuous treatment with the selective PPARγ antagonist GW9662 or vehicle at a rate of 700 ng/kg/min for 4 weeks using subcutaneously implanted osmotic mini-pumps. Additionally, one group of female ApoE(-/-) mice served as a control group. After treatment for 4 weeks mice were sacrificed and read-outs (plaque development, vascular inflammation and insulinsensitivity) were performed.
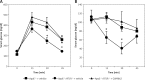
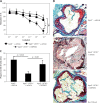
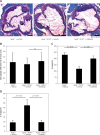
Results: Using AT1R deficient ApoE(-/-) mice (ApoE(-/-)/AT1R(-/-) mice) we found decreased cholesterol-induced endothelial dysfunction and atherogenesis compared to ApoE(-/-) mice. Inhibition of PPARγ by application of the specific PPARγ antagonist GW9662 significantly abolished the anti-atherogenic effects of AT1R deficiency in ApoE(-/-)/AT1R(-/-) mice (plaque area as % of control: ApoE(-/-): 39 ±5%; ApoE(-/-)/AT1R(-/-): 17 ±7%, p = 0.044 vs. ApoE(-/-); ApoE(-/-)/AT1R(-/-) + GW9662: 31 ±8%, p = 0.047 vs. ApoE(-/-)/AT1R(-/-)). Focusing on IL6 as a pro-inflammatory humoral marker we detected significantly increased IL-6 levels in GW9662-treated animals (IL-6 in pg/ml: ApoE(-/-): 230 ±16; ApoE(-/-)/AT1R(-/-): 117 ±20, p = 0.01 vs. ApoE(-/-); ApoE(-/-)/AT1R(-/-) + GW9662: 199 ±20, p = 0.01 vs. ApoE(-/-)/AT1R(-/-)), while the anti-inflammatory marker IL-10 was significantly reduced after PPARγ inhibition in GW9662 animals (IL-10 in pg/ml: ApoE(-/-): 18 ±4; ApoE(-/-)/AT1R(-/-): 55 ±12, p = 0.03 vs. ApoE(-/-); ApoE(-/-)/AT1R(-/-) + GW9662: 19 ±4, p = 0.03 vs. ApoE(-/-)/AT1R(-/-)). Metabolic parameters of glucose homeostasis (glucose and insulin tolerance test) were significantly deteriorated in ApoE(-/-)/AT1R(-/-) mice treated with GW9662 as compared to vehicle-treated ApoE(-/-)/AT1R(-/-) mice. Systolic blood pressure and plasma cholesterol levels were similar in all groups.
Conclusions: Genetic disruption of the AT1R attenuates atherosclerosis and improves endothelial function in an ApoE(-/-) mouse model of hypercholesterolemia-induced atherosclerosis via PPARγ, indicating a significant role of PPARγ in reduced vascular inflammation, improvement of insulin sensitivity and atheroprotection of AT1R deficiency.
Keywords: angiotensin II type 1 receptor; atherosclerosis; inflammation; insulin sensitivity; peroxisome proliferator-activated receptor γ.
Figures

References
-
- Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–6. - PubMed
-
- Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–4. - PubMed
-
- Warnholtz A, Nickenig G, Schulz E, et al. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–33. - PubMed
-
- Wassmann S, Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl. 2006;24:S15–21. - PubMed
-
- Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
