Is erythroferrone finally the long sought-after systemic erythroid regulator of iron?
- PMID: 26322167
- PMCID: PMC4549772
- DOI: 10.4331/wjbc.v6.i3.78
Is erythroferrone finally the long sought-after systemic erythroid regulator of iron?
Abstract
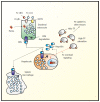
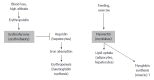
Iron metabolism is regulated on the cellular and the systemic level. Over the last decade, the liver peptide "hepcidin" has emerged as the body's key irons store regulator. The long postulated "erythroid regulator of iron", however, remained elusive. Last year, evidence was provided, that a previously described myokine "myonectin" may also function as the long sought erythroid regulator of iron. Myonectin was therefore re-named "erythroferrone". This editorial provides a brief discussion on the two functions of erythroferrone and also briefly considers the emerging potential role of transferrin receptor 2 in erythropoiesis.
Keywords: Erythroid regulator of iron; Hepcidin; Iron metabolism; Myonectin; Systemic iron regulation.
Figures
References
-
- Lawen A, Lane DJ. Mammalian iron homeostasis in health and disease: uptake, storage, transport, and molecular mechanisms of action. Antioxid Redox Signal. 2013;18:2473–2507. - PubMed
-
- Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci. 2007;44:413–459. - PubMed
-
- Finch C. Regulators of iron balance in humans. Blood. 1994;84:1697–1702. - PubMed
-
- Bothwell TH, Pirzio-Biroli G, Finch CA. Iron absorption. I. Factors influencing absorption. J Lab Clin Med. 1958;51:24–36. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

