Opening the black box of Anaplasma phagocytophilum diversity: current situation and future perspectives
- PMID: 26322277
- PMCID: PMC4536383
- DOI: 10.3389/fcimb.2015.00061
Opening the black box of Anaplasma phagocytophilum diversity: current situation and future perspectives
Abstract
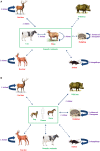
Anaplasma phagocytophilum is a zoonotic obligate intracellular bacterium known to be transmitted by ticks belonging to the Ixodes persulcatus complex. This bacterium can infect several mammalian species, and is known to cause diseases with variable symptoms in many domestic animals. Specifically, it is the causative agent of tick-borne fever (TBF), a disease of important economic impact in European domestic ruminants, and human granulocytic anaplasmosis (HGA), an emerging zoonotic disease in Asia, USA and Europe. A. phagocytophilum epidemiological cycles are complex and involve different ecotypes, vectors, and mammalian host species. Moreover, the epidemiology of A. phagocytophilum infection differs greatly between Europe and the USA. These different epidemiological contexts are associated with considerable variations in bacterial strains. Until recently, few A. phagocytophilum molecular typing tools were available, generating difficulties in completely elucidating the epidemiological cycles of this bacterium. Over the last few years, many A. phagocytophilum typing techniques have been developed, permitting in-depth epidemiological exploration. Here, we review the current knowledge and future perspectives regarding A. phagocytophilum epidemiology and phylogeny, and then focus on the molecular typing tools available for studying A. phagocytophilum genetic diversity.
Keywords: Anaplasma phagocytophilum; diversity; epidemiology; granulocytic anaplasmosis; phylogeny; tick-borne fever; typing technique.
Figures

References
-
- Adams D., Jajosky R., Ajani U., Kriseman J., Sharp P., Onweh D., et al. (2014). Morbidity Mortality Weekly Report Summary of Notifiable Diseases. Available online at: http://www.cdc.gov/mmwr/pdf/wk/mm6153.pdf (Accessed July 21, 2015).
-
- Alberti A., Zobba R., Chessa B., Addis M. F., Sparagano O., Pinna Parpaglia M. L., et al. (2005). Equine and canine Anaplasma phagocytophilum strains isolated on the Island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl. Environ. Microbiol. 71, 6418–6422. 10.1128/AEM.71.10.6418-6422.2005 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

