Thermoanalytical characterization of clindamycin-loaded intravitreal implants prepared by hot melt extrusion
- PMID: 26322295
- PMCID: PMC4549919
- DOI: 10.4103/2277-9175.161563
Thermoanalytical characterization of clindamycin-loaded intravitreal implants prepared by hot melt extrusion
Abstract
Background: The aim of the present study was to evaluate a non-destructive fabrication method in for the development of sustained-release poly (L, D-lactic acid)-based biodegradable clindamycin phosphate implants for the treatment of ocular toxoplasmosis.
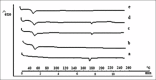
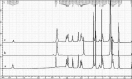
Materials and methods: The rod-shaped intravitreal implants with an average length of 5 mm and a diameter of 0.4 mm were evaluated for their physicochemical parameters. Scanning electron microscopy (SEM), differential scanning calorimetry (DSC), Fourier-transform infrared (FTIR), and nuclear magnetic resonance (1H NMR) studies were employed in order to study the characteristics of these formulations.
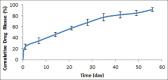
Results: Drug content uniformity test confirmed the uniformity in different implant batches. Furthermore, the DSC, FTIR, and 1H NMR studies proved that the fabrication process did not have any destructive effects either on the drug or on the polymer structures.
Conclusion: These studies showed that the developed sustained-release implants could be of interest for long-term sustained intraocular delivery of clindamycin, which can provide better patient compliance and also have good potential in terms of industrial feasibility.
Keywords: Clindamycin phosphate; D-lactic acid); intravitreal implant; melt extrusion; poly (L.
Conflict of interest statement
Figures




References
-
- Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, et al. Intraocular implants for extended drug delivery: Therapeutic applications. Adv Drug Deliv Rev. 2006;58:1182–202. - PubMed
-
- Choonara YE, Pillay V, Danckwerts MP, Carmichael TR, du Toit LC. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J Pharm Sci. 2010;99:2219–39. - PubMed
-
- Eljarrat-Binstock E, Pe’er J, Domb AJ. New techniques for drug delivery to the posterior eye segment. Pharm Res. 2010;27:530–43. - PubMed
-
- Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13:135–43. - PubMed
-
- Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011;16:270–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

