Translational control by lysine-encoding A-rich sequences
- PMID: 26322332
- PMCID: PMC4552401
- DOI: 10.1126/sciadv.1500154
Translational control by lysine-encoding A-rich sequences
Abstract
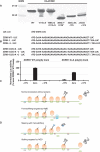
Regulation of gene expression involves a wide array of cellular mechanisms that control the abundance of the RNA or protein products of that gene. Here we describe a gene-regulatory mechanism that is based on poly(A) tracks that stall the translation apparatus. We show that creating longer or shorter runs of adenosine nucleotides, without changes in the amino acid sequence, alters the protein output and the stability of mRNA. Sometimes these changes result in the production of an alternative "frame-shifted" protein product. These observations are corroborated using reporter constructs and in the context of recombinant gene sequences. Approximately two percent of genes in the human genome may be subject to this uncharacterized, yet fundamental form of gene regulation. The potential pool of regulated genes encodes many proteins involved in nucleic acid binding. We hypothesize that the genes we identify are part of a large network whose expression is fine-tuned by poly(A)-tracks, and we provide a mechanism through which synonymous mutations may influence gene expression in pathological states.
Keywords: Lysine; gene regulation; mRNA stability; ribosome stalling; synonymous mutations.
Figures




References
-
- Dinman J. D., Berry M. J., 22 Regulation of Termination and Recoding. Cold Spring Harb. Monogr. Arch. 48, 625–654 (2007); https://cshmonographs.org/index.php/monographs/article/view/3291.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

