Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother's periconceptional diet
- PMID: 26322500
- PMCID: PMC4826136
- DOI: 10.1080/19490976.2015.1086056
Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother's periconceptional diet
Abstract
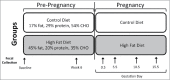
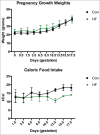
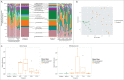
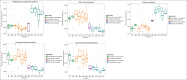
Shifts in the maternal gut microbiome have been implicated in metabolic adaptations to pregnancy. We investigated how pregnancy and diet interact to influence the composition of the maternal gut microbiota. Female C57BL/6 mice were fed either a control or a high fat diet for 8 weeks prior to mating. After confirmation of pregnancy, maternal weight gain and food intake were recorded. Fecal pellets were collected at 2 timepoints prior to mating (at the beginning of the experiment, and after 6 weeks of the specified diet) and at 4 timepoints during pregnancy (gestation day 0.5, 5.5, 10.5, and 15.5). The microbial composition and predicted metabolic functionality of the non-pregnant and pregnant gut was determined via sequencing of the variable 3 region of the 16S rRNA gene. Upon conception, differences in gut microbial communities were observed in both control and high fat-fed mice, including an increase in mucin-degrading bacteria. Control versus high fat-fed pregnant mice possessed the most profound changes to their maternal gut microbiota as indicated by statistically significant taxonomic differences. High fat-fed pregnant mice, when compared to control-fed animals, were found to be significantly enriched in microbes involved in metabolic pathways favoring fatty acid, ketone, vitamin, and bile synthesis. We show that pregnancy-induced changes in the female gut microbiota occur immediately at the onset of pregnancy, are vulnerable to modulation by diet, but are not dependent upon increases in maternal weight gain during pregnancy. High fat diet intake before and during pregnancy results in distinctive shifts in the pregnant gut microbiota in a gestational-age dependent manner and these shifts predict significant differences in the abundance of genes that favor lipid metabolism, glycolysis and gluconeogenic metabolic pathways over the course of pregnancy.
Keywords: gut; high fat diet; intestine; microbiome; obesity; pregnancy.
Figures



References
-
- Organization WH Obesity 2013.
-
- Gluckman PD, Hanson MA. Developmental plasticity and human disease: research directions. J Intern Med 2007; 261:461-71; PMID:17444885; http://dx.doi.org/ 10.1111/j.1365-2796.2007.01802.x - DOI - PubMed
-
- Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG 2005; 112:403-8; PMID:15777435; http://dx.doi.org/ 10.1111/j.1471-0528.2005.00437.x - DOI - PubMed
-
- Patro B, Liber A, Zalewski B, Poston L, Szajewska H, Koletzko B. Maternal and paternal body mass index and offspring obesity: a systematic review. Ann Nutr Metab 2013; 63:32-41; PMID:23887153; http://dx.doi.org/ 10.1159/000350313 - DOI - PubMed
-
- Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Pract Res Clin Endocrinol Metab 2012; 26:627-39; PMID:22980045; http://dx.doi.org/ 10.1016/j.beem.2012.03.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
