Association between mixed rotavirus vaccination types of infants and rotavirus acute gastroenteritis
- PMID: 26322843
- PMCID: PMC5444377
- DOI: 10.1016/j.vaccine.2015.08.027
Association between mixed rotavirus vaccination types of infants and rotavirus acute gastroenteritis
Abstract
Introduction: Rotavirus remains the leading cause of severe diarrhea in children under 5 years worldwide. In the US, Rotarix (RV1) and RotaTeq (RV5), have been associated with reductions in and severity of rotavirus disease. Studies have evaluated the impact of RV1 or RV5 but little is known about the impact of incomplete or mixed vaccination upon vaccine effectiveness.
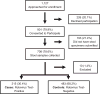
Methods: Case control study to examine association of combined RV1 and RV5 and rotavirus acute gastroenteritis, factoring severity of diarrheal disease. Children born after March 1, 2009 with acute gastroenteritis from three pediatric hospitals in Atlanta, Georgia were approached for enrollment. Survey was administered, stool specimen was collected, and vaccination records were obtained.
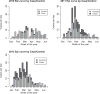
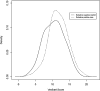
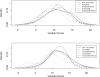
Results: 891 of 1127 children with acute gastroenteritis were enrolled. Stool specimens were collected from 708 for rotavirus testing; 215 stool samples tested positively for rotavirus. Children >12 months of age were more likely to have rotavirus. Children categorized with Vesikari score of >11 were almost twice as likely to be rotavirus positive. Prior rotavirus vaccination decreased the mean Vesikari score, p<0.0001. Children with complete single type vaccination were protected against rotavirus (OR 0.21, 95% CI: 0.14-0.31, p<0.0001).
Conclusion: Complete rotavirus vaccination with a single vaccine type resulted in protection against rotavirus diarrhea and decrease in severity of rotavirus gastroenteritis. Incomplete rotavirus vaccination either with a single vaccine or mixed vaccination types also provided some protection.
Keywords: Acute gastroenteritis; Pediatric; Rotavirus; Rotavirus vaccination.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
-
- Bernstein DI. Rotavirus overview. Pediatr Infect Dis J. 2009;28:S50–3. - PubMed
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. - PubMed
-
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–32. - PubMed
-
- Cortese M, Parashar U, Centers for Disease Control and Prevention (CDC) Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58:1–25. - PubMed
-
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pen-tavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010;125:e208–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

