Immunotherapy for Lewis lung carcinoma utilizing dendritic cells infected with CK19 gene recombinant adenoviral vectors
- PMID: 26323510
- PMCID: PMC4583529
- DOI: 10.3892/or.2015.4231
Immunotherapy for Lewis lung carcinoma utilizing dendritic cells infected with CK19 gene recombinant adenoviral vectors
Abstract
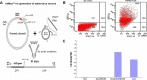
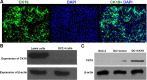
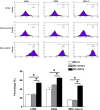
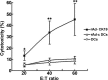
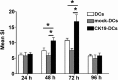
Dendritic cells (DCs) as 'professional' antigen-presenting cells (APCs) initiate and regulate immune responses to various antigens. DC-based vaccines have become a promising modality in cancer immunotherapy. Cytokeratin 19 (CK19) protein is expressed at high levels in lung cancer and many other tumor cells, suggesting CK19 as a potential tumor‑specific target for cancer immune therapy. We constructed a recombinant adenoviral vector containing the CK19 gene (rAd-CK19). DCs transfected with rAd-CK19 were used to vaccinate C57BL/6 mice bearing xenografts derived from Lewis lung carcinoma (LLC) cells. The transfected DCs gave rise to potent CK19-specific cytotoxic T lymphocytes (CTLs) capable of lysing LLC cells. Mice immunized with the rAd‑CK19-DCs exhibited significantly attenuated tumor growth (including tumor volume and weight) when compared to the tumor growth of mice immunized with rAd-c DCs or DCs during the 24-day observation period (P<0.05). The results revealed that the mice vaccinated with the rAd-CK19-DCs exhibited a potent protective and therapeutic antitumor immunity to LLC cells in the subcutaneous model along with an inhibitive effect on tumor growth compared to the mice vaccinated with the rAd-c DCs or DCs alone. The present study proposes a meaningful mode of action utilizing rAd-CK19 DCs in lung cancer immunotherapy.
Figures

References
-
- Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: Diagnosis and management. Am Fam Physician. 2007;75:56–63. - PubMed
-
- Moscatello DK, Ramirez G, Wong AJ. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997;57:1419–1424. - PubMed
-
- Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9:4247–4254. - PubMed
-
- Ciesielski MJ, Kazim AL, Barth RF, Fenstermaker RA. Cellular antitumor immune response to a branched lysine multiple antigenic peptide containing epitopes of a common tumor-specific antigen in a rat glioma model. Cancer Immunol Immunother. 2005;54:107–119. doi: 10.1007/s00262-004-0576-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

