Silica bioreplication preserves three-dimensional spheroid structures of human pluripotent stem cells and HepG2 cells
- PMID: 26323570
- PMCID: PMC4555166
- DOI: 10.1038/srep13635
Silica bioreplication preserves three-dimensional spheroid structures of human pluripotent stem cells and HepG2 cells
Abstract
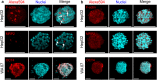
Three-dimensional (3D) cell cultures produce more in vivo-like multicellular structures such as spheroids that cannot be obtained in two-dimensional (2D) cell cultures. Thus, they are increasingly employed as models for cancer and drug research, as well as tissue engineering. It has proven challenging to stabilize spheroid architectures for detailed morphological examination. Here we overcome this issue using a silica bioreplication (SBR) process employed on spheroids formed from human pluripotent stem cells (hPSCs) and hepatocellular carcinoma HepG2 cells cultured in the nanofibrillar cellulose (NFC) hydrogel. The cells in the spheroids are more round and tightly interacting with each other than those in 2D cultures, and they develop microvilli-like structures on the cell membranes as seen in 2D cultures. Furthermore, SBR preserves extracellular matrix-like materials and cellular proteins. These findings provide the first evidence of intact hPSC spheroid architectures and similar fine structures to 2D-cultured cells, providing a pathway to enable our understanding of morphogenesis in 3D cultures.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

