Annexin A2-dependent actin bundling promotes secretory granule docking to the plasma membrane and exocytosis
- PMID: 26323692
- PMCID: PMC4555831
- DOI: 10.1083/jcb.201412030
Annexin A2-dependent actin bundling promotes secretory granule docking to the plasma membrane and exocytosis
Abstract
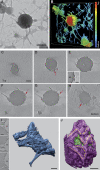
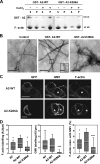
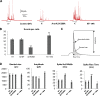
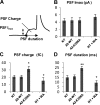
Annexin A2, a calcium-, actin-, and lipid-binding protein involved in exocytosis, mediates the formation of lipid microdomains required for the structural and spatial organization of fusion sites at the plasma membrane. To understand how annexin A2 promotes this membrane remodeling, the involvement of cortical actin filaments in lipid domain organization was investigated. 3D electron tomography showed that cortical actin bundled by annexin A2 connected docked secretory granules to the plasma membrane and contributed to the formation of GM1-enriched lipid microdomains at the exocytotic sites in chromaffin cells. When an annexin A2 mutant with impaired actin filament-bundling activity was expressed, the formation of plasma membrane lipid microdomains and the number of exocytotic events were decreased and the fusion kinetics were slower, whereas the pharmacological activation of the intrinsic actin-bundling activity of endogenous annexin A2 had the opposite effects. Thus, annexin A2-induced actin bundling is apparently essential for generating active exocytotic sites.
© 2015 Gabel et al.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

