Origin of eukaryotes from within archaea, archaeal eukaryome and bursts of gene gain: eukaryogenesis just made easier?
- PMID: 26323764
- PMCID: PMC4571572
- DOI: 10.1098/rstb.2014.0333
Origin of eukaryotes from within archaea, archaeal eukaryome and bursts of gene gain: eukaryogenesis just made easier?
Abstract
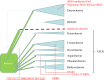
The origin of eukaryotes is a fundamental, forbidding evolutionary puzzle. Comparative genomic analysis clearly shows that the last eukaryotic common ancestor (LECA) possessed most of the signature complex features of modern eukaryotic cells, in particular the mitochondria, the endomembrane system including the nucleus, an advanced cytoskeleton and the ubiquitin network. Numerous duplications of ancestral genes, e.g. DNA polymerases, RNA polymerases and proteasome subunits, also can be traced back to the LECA. Thus, the LECA was not a primitive organism and its emergence must have resulted from extensive evolution towards cellular complexity. However, the scenario of eukaryogenesis, and in particular the relationship between endosymbiosis and the origin of eukaryotes, is far from being clear. Four recent developments provide new clues to the likely routes of eukaryogenesis. First, evolutionary reconstructions suggest complex ancestors for most of the major groups of archaea, with the subsequent evolution dominated by gene loss. Second, homologues of signature eukaryotic proteins, such as actin and tubulin that form the core of the cytoskeleton or the ubiquitin system, have been detected in diverse archaea. The discovery of this 'dispersed eukaryome' implies that the archaeal ancestor of eukaryotes was a complex cell that might have been capable of a primitive form of phagocytosis and thus conducive to endosymbiont capture. Third, phylogenomic analyses converge on the origin of most eukaryotic genes of archaeal descent from within the archaeal evolutionary tree, specifically, the TACK superphylum. Fourth, evidence has been presented that the origin of the major archaeal phyla involved massive acquisition of bacterial genes. Taken together, these findings make the symbiogenetic scenario for the origin of eukaryotes considerably more plausible and the origin of the organizational complexity of eukaryotic cells more readily explainable than they appeared until recently.
Keywords: archaea; cytoskeleton; endosymbiosis; horizontal gene transfer; phagocytosis.
© 2015 The Authors.
Figures
References
-
- Lane N. 2015. The vital question: energy, evolution, and the origins of complex life. London, UK: W.W. Norton & Company.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
