Design and synthesis of novel bivalent ligands (MOR and DOR) by conjugation of enkephalin analogues with 4-anilidopiperidine derivatives
- PMID: 26323872
- PMCID: PMC4642889
- DOI: 10.1016/j.bmcl.2015.07.064
Design and synthesis of novel bivalent ligands (MOR and DOR) by conjugation of enkephalin analogues with 4-anilidopiperidine derivatives
Abstract
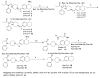
We describe the design and synthesis of novel bivalent ligands based on the conjugation of 4-anilidopiperidine derivatives with enkephalin analogues. The design of non-peptide analogues is explored with 5-amino substituted (tetrahydronaphthalen-2yl) methyl containing 4-anilidopiperidine derivatives, while non-peptide-peptide ligands are explored by conjugating the C-terminus of enkephalin analogues (H-Xxx-DAla-Gly-Phe-OH) to the amino group of 4-anilidopiperidine small molecule derivatives with and without a linker. These novel bivalent ligands are evaluated for biological activities at μ and δ opioid receptors. They exhibit very good affinities at μ and δ opioid receptors, and potent agonist activities in MVD and GPI assays. Among these the lead bivalent ligand 17 showed excellent binding affinities (0.1 nM and 0.5 nM) at μ and δ opioid receptors respectively, and was found to have very potent agonist activities in MVD (56 ± 5.9 nM) and GPI (4.6 ± 1.9 nM) assays. In vivo the lead bivalent ligand 17 exhibited a short duration of action (<15 min) comparable to 4-anilidopiperidine derivatives, and moderate analgesic activity. The ligand 17 has limited application against acute pain but may have utility in settings where a highly reversible analgesic is required.
Keywords: Bivalent ligands; Enkephalins; Opioid receptors; Opioids.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
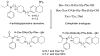

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

