Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum
- PMID: 26323940
- PMCID: PMC4553924
- DOI: 10.1186/s13104-015-1388-1
Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum
Abstract
Background: The present investigation evaluated 4 different solvent compositions for their relative capacity to extract total phenolic and total flavonoid (TF) components of the leaves, trunks, and stems of Bucida buceras L. (Combretaceae), and the stems of Phoradendron californicum (Viscaceae), plus mesquite and oak species endemic to the Southwestern United States, northern Mexico, and tropical regions of Central and South America, as well as to profile the composition of these plant materials and to measure their antioxidant capacity.
Methods: The total phenolic content of plant material used in the present investigation was measured using the Folin-Ciocalteau assay. Total flavonoids were assayed by AlCl3 and 2,4-dinitrophenylhydrazin colorimetry. Nitroblue tetrazolium was utilized for scavenging of superoxide anion, and in vitro antioxidant activity was evaluated using the 2, 2-diphenyl-1-picrylhydrazyl and Ferric Reducing/Antioxidant Power assays.
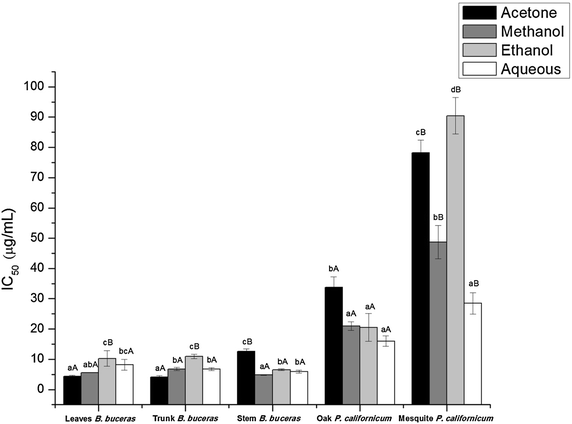
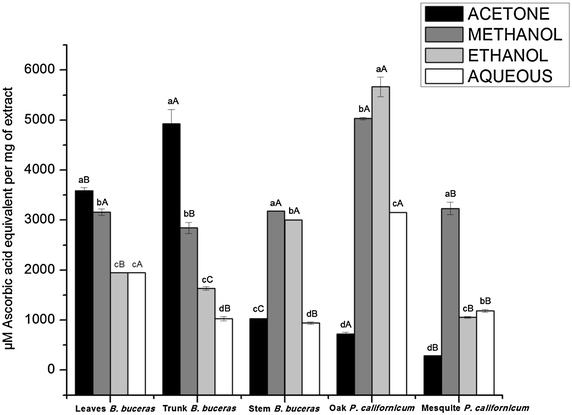
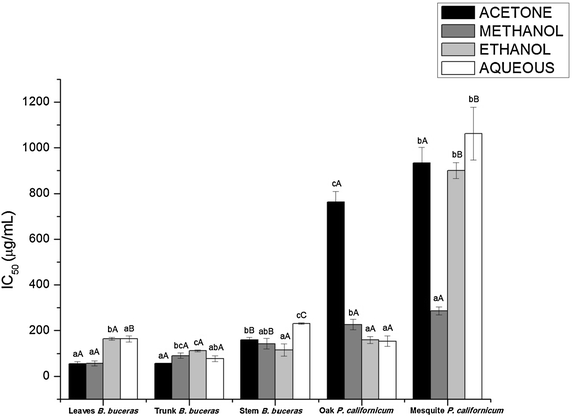
Results: Phytochemical screening of each plant extract evaluated revealed the following major results: (1) No evidence of alkaloids for each of the extraction phases tested was detected in the hexanic, ethanolic, or aqueous phases of Bucida buceras and Phoradendron californicum (oak and mesquite); (2) Analysis of the hexane phase of B. buceras and P. californicum (mesquite) extracts revealed the presence of carotenes, triterpenes/steroids, and lactonic groups; (3) Analysis of the ethanol and aqueous extraction phases for both plants revealed the presence of a diverse range of compounds, including tripterpenes/steroids, lactonics groups, saponins, phenols/tannins, amines and/or amino acids, and flavonoids/anthocyanins; and (4) The highest total phenolic and flavonoid content were observed in P. californicum (oak): 523.886 ± 51.457 µg GAE/mg extract and 409.651 ± 23.091 µg/mg of extract for methanol and aqueous fractions, respectively. The highest flavonoid content was 237.273 ± 21.250 µg PNE/mg extract in the acetone extract of Bucida buceras stems; while the flavonol content (260.685 ± 23.031 µg CE/mg extract) was higher in the ethanol extract of P. californicum (oak). The acetone extract of B. buceras trunk extract showed the highest levels of DPPH radical-scavenging activity (IC50 = 4.136 ± 0.446 µg/mL) and reducing power (4928.392 ± 281.427 µM AAE/mg extract). The highest superoxide radical scavenging activity (IC50) was 55.249 ± 9.829 µg/mL, observed in acetone extracts of B. buceras leaves.
Conclusions: The results of the present investigation demonstrated the effects of extraction solvent on phenolic and flavonoid content yield-and antioxidant activities by Bucida buceras and Phoradendron californicum. The present investigation further revealed that Bucida buceras exhibited optimal antioxidant capacity when acetone was used as extraction solvent; and the highest yield of phenols and flavonoids were obtained from the P. californicum oak, using methanol and aqueous solvents, respectively.
Figures

Similar articles
-
Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds.BMC Complement Altern Med. 2014 Feb 4;14:45. doi: 10.1186/1472-6882-14-45. BMC Complement Altern Med. 2014. PMID: 24495381 Free PMC article.
-
Retino-protective effect of Bucida buceras against oxidative stress induced by H2O2 in human retinal pigment epithelial cells line.BMC Complement Altern Med. 2015 Jul 29;15:254. doi: 10.1186/s12906-015-0765-6. BMC Complement Altern Med. 2015. PMID: 26219933 Free PMC article.
-
Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts.Molecules. 2023 Feb 28;28(5):2263. doi: 10.3390/molecules28052263. Molecules. 2023. PMID: 36903521 Free PMC article.
-
Consistency of Phenolic Compounds in Plant Residues Parts: A Review of Primary Sources, Key Compounds, and Extraction Trends.J Agric Food Chem. 2025 May 14;73(19):11515-11534. doi: 10.1021/acs.jafc.5c01868. Epub 2025 Apr 29. J Agric Food Chem. 2025. PMID: 40300049 Free PMC article. Review.
-
Recent advances in the combination of organic solvent-free extraction, chemical standardization, antioxidant assay, and cell culture metabolomics for functional food and its by-product.Crit Rev Food Sci Nutr. 2024 Nov;64(32):11919-11933. doi: 10.1080/10408398.2023.2245040. Epub 2023 Aug 13. Crit Rev Food Sci Nutr. 2024. PMID: 37574586 Review.
Cited by
-
Antimicrobial Activity of Euphorbia helioscopia L. Against Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro.Cureus. 2024 Sep 21;16(9):e69840. doi: 10.7759/cureus.69840. eCollection 2024 Sep. Cureus. 2024. PMID: 39435254 Free PMC article.
-
Metabolomic Characterization of Phoradendron brachystachyum Mistletoe and In-Silico and In-Vitro Investigation of Its Therapeutic Potential in Metabolic Disorders.Plants (Basel). 2023 Jul 22;12(14):2729. doi: 10.3390/plants12142729. Plants (Basel). 2023. PMID: 37514343 Free PMC article.
-
Anti-melanogenic activity of Myristica fragrans extract against Aspergillus fumigatus using phenotypic based screening.BMC Complement Med Ther. 2020 Mar 2;20(1):67. doi: 10.1186/s12906-020-2859-z. BMC Complement Med Ther. 2020. PMID: 32122336 Free PMC article.
-
Facile production of chlorophyllides using recombinant CrCLH1 and their cytotoxicity towards multidrug resistant breast cancer cell lines.PLoS One. 2021 Apr 30;16(4):e0250565. doi: 10.1371/journal.pone.0250565. eCollection 2021. PLoS One. 2021. PMID: 33930043 Free PMC article.
-
Modulation of Inflammatory Cytokines and Islet Morphology as Therapeutic Mechanisms of Basella alba in Streptozotocin-Induced Diabetic Rats.Toxicol Res. 2018 Oct;34(4):325-332. doi: 10.5487/TR.2018.34.4.325. Epub 2018 Oct 15. Toxicol Res. 2018. PMID: 30370007 Free PMC article.
References
-
- Mazandarani M, Zarghami P, Zolfaghari M, Ghaemi E, Bayat H. Effects of solvent type on phenolics and flavonoids content and antioxidant activities in Onosma dichroanthum Boiss. J Med Plants Res. 2012;6(28):4481–4488. doi: 10.5897/JMPR11.1460. - DOI
-
- Sikwese F, Duodo K. Antioxidant effects of crude phenolic extracts from sorghum bran in sunflower oil in the presence of ferric ions. Food Chem. 2007;104:324–331. doi: 10.1016/j.foodchem.2006.11.042. - DOI
-
- Behere B, Verma N, Sonone A, Makhija U. Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT-Food Sci Technol. 2006;39:80–85. doi: 10.1016/j.lwt.2004.11.007. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

