Development of Small-molecule HIV Entry Inhibitors Specifically Targeting gp120 or gp41
- PMID: 26324044
- PMCID: PMC4775441
- DOI: 10.2174/1568026615666150901114527
Development of Small-molecule HIV Entry Inhibitors Specifically Targeting gp120 or gp41
Abstract
Human immunodeficiency virus type 1 (HIV-1) envelope (Env) glycoprotein surface subunit gp120 and transmembrane subunit gp41 play important roles in HIV-1 entry, thus serving as key targets for the development of HIV-1 entry inhibitors. T20 peptide (enfuvirtide) is the first U.S. FDA-approved HIV entry inhibitor; however, its clinical application is limited by the lack of oral availability. Here, we have described the structure and function of the HIV-1 gp120 and gp41 subunits and reviewed advancements in the development of small-molecule HIV entry inhibitors specifically targeting these two Env glycoproteins. We then compared the advantages and disadvantages of different categories of HIV entry inhibitor candidates and further predicted the future trend of HIV entry inhibitor development.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
Figures

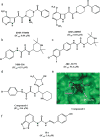
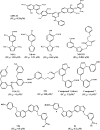


References
-
- Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Schooley RT. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317(4):185–191. - PubMed
-
- Brook I. Approval of zidovudine (AZT) for acquired immunodeficiency syndrome. A challenge to the medical and pharmaceutical communities. JAMA. 1987;258(11):1517. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT, Jonas L, Meibohm A, Emini EA, Chodakewitz JA, Deutsch P, Holder D, Schleif WA, Condra JH. Treatment with Indinavir, Zidovudine, and Lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–739. - PubMed
-
- Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387(6629):188–191. - PubMed
-
- New York Blood Center. Synthetic peptides as inhibitors of HIV-1. 1992 US5,444,044.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
