One-Step Synthesis of Chiral Oxindole-type Analogues with Potent Anti-inflammatory and Analgesic Activities
- PMID: 26324065
- PMCID: PMC4555174
- DOI: 10.1038/srep13699
One-Step Synthesis of Chiral Oxindole-type Analogues with Potent Anti-inflammatory and Analgesic Activities
Abstract
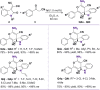
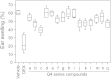
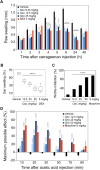
Here we report a facile approach to synthesize highly optically active oxindole-type analogues with both high yield and enantioselectivity. This single-step synthesis strategy represents a substantial improvement upon existing methods that are often involved with multi-step routes and have suboptimal atomic economy. One such compound, namely Q4c, showed remarkable in vivo anti-inflammatory activity with efficiency approaching to that of a steroidal compound dexamethasone. Moreover, Q4c alleviated pain in mouse models with comparable activity to morphine. Further investigation suggested that nitric oxide signaling pathway is involved in the anti-inflammatory and analgesic activities of Q4c. Notably, this is the first time that chiral oxindole-type analogues have been identified to be both anti-inflammatory and analgesic, and our study also paved the way for future development of oxindoles as drug candidates in this field.
Figures


References
-
- Smith W. L. & Dewitt D. L. Prostaglandin endoperoxide H synthases-1 and -2. Adv. Immunol. 62, 167–215 (1996). - PubMed
-
- Ding K. et al. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 127, 10130–10131 (2005). - PubMed
-
- Roth S. H. Coming to terms with nonsteroidal anti-inflammatory drug gastropathy. Drugs 72, 873–879 (2012). - PubMed
-
- Marnett L. J. The COXIB experience: a look in the rearview mirror. Annu. Rev. Pharmacol. Toxicol. 49, 265–290 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

