Octanoylation of early intermediates of mycobacterial methylglucose lipopolysaccharides
- PMID: 26324178
- PMCID: PMC4555173
- DOI: 10.1038/srep13610
Octanoylation of early intermediates of mycobacterial methylglucose lipopolysaccharides
Abstract
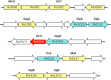
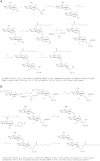
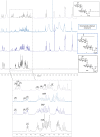
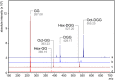
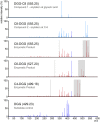
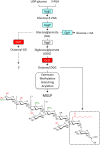
Mycobacteria synthesize unique intracellular methylglucose lipopolysaccharides (MGLP) proposed to modulate fatty acid metabolism. In addition to the partial esterification of glucose or methylglucose units with short-chain fatty acids, octanoate was invariably detected on the MGLP reducing end. We have identified a novel sugar octanoyltransferase (OctT) that efficiently transfers octanoate to glucosylglycerate (GG) and diglucosylglycerate (DGG), the earliest intermediates in MGLP biosynthesis. Enzymatic studies, synthetic chemistry, NMR spectroscopy and mass spectrometry approaches suggest that, in contrast to the prevailing consensus, octanoate is not esterified to the primary hydroxyl group of glycerate but instead to the C6 OH of the second glucose in DGG. These observations raise important new questions about the MGLP reducing end architecture and about subsequent biosynthetic steps. Functional characterization of this unique octanoyltransferase, whose gene has been proposed to be essential for M. tuberculosis growth, adds new insights into a vital mycobacterial pathway, which may inspire new drug discovery strategies.
Figures

References
-
- Koul A., Arnoult E., Lounis N., Guillemont J. & Andries K. The challenge of new drug discovery for tuberculosis. Nature 469, 483–490 (2011). - PubMed
-
- Daffé M., Crick D. C. & Jackson M. in Molecular Genetics of Mycobacteria, Second Edition (American Society of Microbiology, 2014).
-
- Mendes V., Maranha A., Alarico S. & Empadinhas N. Biosynthesis of mycobacterial methylglucose lipopolysaccharides. Nat Prod Rep 29, 834–844 (2012). - PubMed
-
- Xia L., Zheng R. B. & Lowary T. L. Revisiting the specificity of an alpha-(1–>4)-mannosyltransferase involved in mycobacterial methylmannose polysaccharide biosynthesis. Chembiochem 13, 1139–1151 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

