Inhibitory effects of magnolol and honokiol on human calcitonin aggregation
- PMID: 26324190
- PMCID: PMC4555095
- DOI: 10.1038/srep13556
Inhibitory effects of magnolol and honokiol on human calcitonin aggregation
Abstract
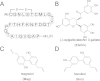
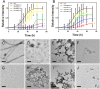
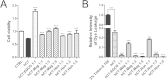
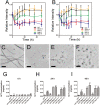
Amyloid formation is associated with multiple amyloidosis diseases. Human calcitonin (hCT) is a typical amyloidogenic peptide, its aggregation is associated with medullary carcinoma of the thyroid (MTC), and also limits its clinical application. Magnolia officinalis is a traditional Chinese herbal medicine; its two major polyphenol components, magnolol (Mag) and honokiol (Hon), have displayed multiple functions. Polyphenols like flavonoids and their derivatives have been extensively studied as amyloid inhibitors. However, the anti-amyloidogenic property of a biphenyl backbone containing polyphenols such as Mag and Hon has not been reported. In this study, these two compounds were tested for their effects on hCT aggregation. We found that Mag and Hon both inhibited the amyloid formation of hCT, whereas Mag showed a stronger inhibitory effect; moreover, they both dose-dependently disassembled preformed hCT aggregates. Further immuno-dot blot and dynamic light scattering studies suggested Mag and Hon suppressed the aggregation of hCT both at the oligomerization and the fibrillation stages, while MTT-based and dye-leakage assays demonstrated that Mag and Hon effectively reduced cytotoxicity caused by hCT aggregates. Furthermore, isothermal titration calorimetry indicated Mag and Hon both interact with hCT. Together, our study suggested a potential anti-amyloidogenic property of these two compounds and their structure related derivatives.
Figures



References
-
- Kelly J. W. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol 8, 101–6 (1998). - PubMed
-
- Stefani M. & Dobson C. M. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med (Berl) 81, 678–99 (2003). - PubMed
-
- Bucciantini M. et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416, 507–11 (2002). - PubMed
-
- Chiti F. & Dobson C. M. Protein misfolding, functional amyloid, and human disease. Annual Review of Biochemistry 75, 333–366 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

