Analysis of the Zidovudine Resistance Mutations T215Y, M41L, and L210W in HIV-1 Reverse Transcriptase
- PMID: 26324274
- PMCID: PMC4649230
- DOI: 10.1128/AAC.05069-14
Analysis of the Zidovudine Resistance Mutations T215Y, M41L, and L210W in HIV-1 Reverse Transcriptase
Abstract
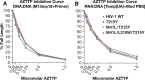
Although anti-human immunodeficiency virus type 1 (HIV-1) therapies have become more sophisticated and more effective, drug resistance continues to be a major problem. Zidovudine (azidothymidine; AZT) was the first nucleoside reverse transcriptase (RT) inhibitor (NRTI) approved for the treatment of HIV-1 infections and is still being used, particularly in the developing world. This drug targets the conversion of single-stranded RNA to double-stranded DNA by HIV-1 RT. However, resistance to the drug quickly appeared both in viruses replicating in cells in culture and in patients undergoing AZT monotherapy. The primary resistance pathway selects for mutations of T215 that change the threonine to either a tyrosine or a phenylalanine (T215Y/F); this resistance pathway involves an ATP-dependent excision mechanism. The pseudo-sugar ring of AZT lacks a 3' OH; RT incorporates AZT monophosphate (AZTMP), which blocks the end of the viral DNA primer. AZT-resistant forms of HIV-1 RT use ATP in an excision reaction to unblock the 3' end of the primer strand, allowing its extension by RT. The T215Y AZT resistance mutation is often accompanied by two other mutations, M41L and L210W. In this study, the roles of these mutations, in combination with T215Y, were examined to determine whether they affect polymerization and excision by HIV-1 RT. The M41L mutation appears to help restore the DNA polymerization activity of RT containing the T215Y mutation and also enhances AZTMP excision. The L210W mutation plays a similar role, but it enhances excision by RTs that carry the T215Y mutation when ATP is present at a low concentration.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Hughes SH. 2015. Reverse transcription of retroviruses and LTR retrotransposons, p 1051–1070. In Craig NL, Chandler M, Gellert M, Lambowitz AM, Rice PA, Sandmeyer S (ed), Mobile DNA III. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

