NagR Differentially Regulates the Expression of the glmS and nagAB Genes Required for Amino Sugar Metabolism by Streptococcus mutans
- PMID: 26324448
- PMCID: PMC4621086
- DOI: 10.1128/JB.00606-15
NagR Differentially Regulates the Expression of the glmS and nagAB Genes Required for Amino Sugar Metabolism by Streptococcus mutans
Abstract
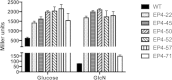
The ability of bacteria to metabolize glucosamine (GlcN) and N-acetyl-d-glucosamine (GlcNAc) is considered important for persistent colonization of the oral cavity. In the dental caries pathogen Streptococcus mutans, the NagR protein regulates the expression of glmS, which encodes a GlcN-6-P synthetase, and nagA (GlcNAc-6-P deacetylase) and nagB (GlcN-6-P deaminase), which are required for the catabolism of GlcNAc and GlcN. Two NagR-binding sites (dre) were identified in each of the promoter regions for nagB and glmS. Using promoter-reporter gene fusions, the role of each dre site was examined in the regulation of glmS and nagB promoter activities in cells grown with glucose, GlcNAc, or GlcN. A synergistic relationship between the two dre sites in the glmS promoter that required proper spacing was observed, but that was not the case for nagB. Binding of purified NagR to DNA fragments from both promoter regions, as well as to dre sites alone, was strongly influenced by particular sugar phosphates. Using a random mutagenesis approach that targeted the effector-binding domain of NagR, mutants that displayed aberrant regulation of both the glmS and nagAB genes were identified. Collectively, these findings provide evidence that NagR is essential for regulation of genes for both the synthesis and catabolism of GlcN and GlcNAc in S. mutans, and that NagR engages differently with the target promoter regions in response to specific metabolites interacting with the effector-binding domain of NagR.
Importance: Glucosamine and N-acetylglucosamine are among the most abundant naturally occurring sugars on the planet, and they are catabolized by many bacterial species as sources of carbon and nitrogen. Representing a group called lactic acid bacteria (LAB), the human dental caries pathogen Streptococcus mutans is shown to differ from known paradigm organisms in that it possesses a GntR/HutC-type regulator, NagR, that is required for the regulation of both catabolism of GlcN and biosynthesis. Results reported here reveal a simple and elegant mechanism whereby NagR differentially regulates two opposing biological processes by surveying metabolic intermediates. This study provides insights that may contribute to the development of novel therapeutic tools to combat dental caries and other infectious diseases.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

