Integrins mediate mechanical compression-induced endothelium-dependent vasodilation through endothelial nitric oxide pathway
- PMID: 26324675
- PMCID: PMC4555471
- DOI: 10.1085/jgp.201411350
Integrins mediate mechanical compression-induced endothelium-dependent vasodilation through endothelial nitric oxide pathway
Abstract
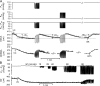
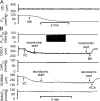
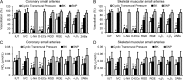
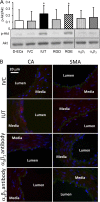
Cardiac and skeletal muscle contraction lead to compression of intramuscular arterioles, which, in turn, leads to their vasodilation (a process that may enhance blood flow during muscle activity). Although endothelium-derived nitric oxide (NO) has been implicated in compression-induced vasodilation, the mechanism whereby arterial compression elicits NO production is unclear. We cannulated isolated swine (n = 39) myocardial (n = 69) and skeletal muscle (n = 60) arteriole segments and exposed them to cyclic transmural pressure generated by either intraluminal or extraluminal pressure pulses to simulate compression in contracting muscle. We found that the vasodilation elicited by internal or external pressure pulses was equivalent; moreover, vasodilation in response to pressure depended on changes in arteriole diameter. Agonist-induced endothelium-dependent and -independent vasodilation was used to verify endothelial and vascular smooth muscle cell viability. Vasodilation in response to cyclic changes in transmural pressure was smaller than that elicited by pharmacological activation of the NO signaling pathway. It was attenuated by inhibition of NO synthase and by mechanical removal of the endothelium. Stemming from previous observations that endothelial integrin is implicated in vasodilation in response to shear stress, we found that function-blocking integrin α5β1 or αvβ3 antibodies attenuated cyclic compression-induced vasodilation and NOx (NO(-)2 and NO(-)3) production, as did an RGD peptide that competitively inhibits ligand binding to some integrins. We therefore conclude that integrin plays a role in cyclic compression-induced endothelial NO production and thereby in the vasodilation of small arteries during cyclic transmural pressure loading.
© 2015 Lu and Kassab.
Figures



Similar articles
-
Integrin signaling transduces shear stress--dependent vasodilation of coronary arterioles.Circ Res. 1997 Mar;80(3):320-6. doi: 10.1161/01.res.80.3.320. Circ Res. 1997. PMID: 9048651
-
Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries.J Appl Physiol (1985). 2005 Mar;98(3):940-6. doi: 10.1152/japplphysiol.00408.2004. Epub 2004 Nov 5. J Appl Physiol (1985). 2005. PMID: 15531569
-
Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries.J Physiol. 2006 Apr 15;572(Pt 2):561-7. doi: 10.1113/jphysiol.2005.099507. Epub 2006 Feb 23. J Physiol. 2006. PMID: 16497720 Free PMC article.
-
[Role of endothelial nitric oxide in the regulation of the vasomotor system].Pathol Biol (Paris). 1998 Mar;46(3):181-9. Pathol Biol (Paris). 1998. PMID: 9769914 Review. French.
-
[Nitric oxide: the endogenous nitrate in the cardiovascular system].Herz. 1996 Jun;21 Suppl 1:50-60. Herz. 1996. PMID: 8767925 Review. German.
Cited by
-
Cell Death in Pulmonary Arterial Hypertension.Int J Med Sci. 2024 Jul 14;21(10):1840-1851. doi: 10.7150/ijms.93902. eCollection 2024. Int J Med Sci. 2024. PMID: 39113898 Free PMC article. Review.
-
Pressure-induced vasodilation mimicking vasculopathy in a pediatric patient.JAAD Case Rep. 2016 Feb 27;2(1):87-8. doi: 10.1016/j.jdcr.2015.12.004. eCollection 2016 Jan. JAAD Case Rep. 2016. PMID: 27051838 Free PMC article. No abstract available.
-
Skeletal muscle contraction-induced vasodilation in the microcirculation.J Exerc Rehabil. 2017 Oct 30;13(5):502-507. doi: 10.12965/jer.1735114.557. eCollection 2017 Oct. J Exerc Rehabil. 2017. PMID: 29114523 Free PMC article. Review.
-
The machinery of healthy vasoconstriction: an overview.Pflugers Arch. 2025 Jul 11. doi: 10.1007/s00424-025-03103-6. Online ahead of print. Pflugers Arch. 2025. PMID: 40640430 Review.
-
Swimming motions evoke Ca2+ events in vascular endothelial cells of larval zebrafish via mechanical activation of Piezo1.bioRxiv [Preprint]. 2025 Feb 8:2025.02.05.636757. doi: 10.1101/2025.02.05.636757. bioRxiv. 2025. PMID: 39975374 Free PMC article. Preprint.
References
-
- Awolesi M.A., Widmann M.D., Sessa W.C., and Sumpio B.E.. 1994. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 116:439–444, discussion:444–445. - PubMed
-
- Buckwalter J.B., Ruble S.B., Mueller P.J., and Clifford P.S.. 1998. Skeletal muscle vasodilation at the onset of exercise. J. Appl. Physiol. 85:1649–1654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

