Pertussis Toxin Exploits Specific Host Cell Signaling Pathways for Promoting Invasion and Translocation of Escherichia coli K1 RS218 in Human Brain-derived Microvascular Endothelial Cells
- PMID: 26324705
- PMCID: PMC4598994
- DOI: 10.1074/jbc.M115.650101
Pertussis Toxin Exploits Specific Host Cell Signaling Pathways for Promoting Invasion and Translocation of Escherichia coli K1 RS218 in Human Brain-derived Microvascular Endothelial Cells
Abstract
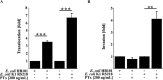
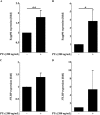
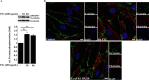
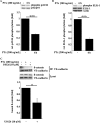
Pertussis toxin (PTx), an AB5 toxin and major virulence factor of the whooping cough-causing pathogen Bordetella pertussis, has been shown to affect the blood-brain barrier. Dysfunction of the blood-brain barrier may facilitate penetration of bacterial pathogens into the brain, such as Escherichia coli K1 (RS218). In this study, we investigated the influence of PTx on blood-brain barrier permissiveness to E. coli infection using human brain-derived endothelial HBMEC and TY10 cells as in vitro models. Our results indicate that PTx acts at several key points of host cell intracellular signaling pathways, which are also affected by E. coli K1 RS218 infection. Application of PTx increased the expression of the pathogen binding receptor gp96. Further, we found an activation of STAT3 and of the small GTPase Rac1, which have been described as being essential for bacterial invasion involving host cell actin cytoskeleton rearrangements at the bacterial entry site. In addition, we showed that PTx induces a remarkable relocation of VE-cadherin and β-catenin from intercellular junctions. The observed changes in host cell signaling molecules were accompanied by differences in intracellular calcium levels, which might act as a second messenger system for PTx. In summary, PTx not only facilitates invasion of E. coli K1 RS218 by activating essential signaling cascades; it also affects intercellular barriers to increase paracellular translocation.
Keywords: Bordetella pertussis; Escherichia coli (E. coli); VE-cadherin; beta-catenin (B-catenin); endothelial dysfunction; pertussis toxin; translocation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Geier D. A., Geier M. R. (2004) An evaluation of serious neurological disorders following immunization: a comparison of whole-cell pertussis and acellular pertussis vaccines. Brain Dev. 26, 296–300 - PubMed
-
- Heininger U., Kleemann W. J., Cherry J. D., and Sudden Infant Death Syndrome Study Group (2004) A controlled study of the relationship between Bordetella pertussis infections and sudden unexpected deaths among German infants. Pediatrics 114, e9–e15 - PubMed
-
- Grant C. C., McKay E. J., Simpson A., Buckley D. (1998) Pertussis encephalopathy with high cerebrospinal fluid antibody titers to pertussis toxin and filamentous hemagglutinin. Pediatrics 102, 986–990 - PubMed
-
- Seidel G., Böcker K., Schulte J., Wewer C., Greune L., Humberg V., Schmidt M. A. (2011) Pertussis toxin permeabilization enhances the traversal of Escherichia coli K1, macrophages, and monocytes in a cerebral endothelial barrier model in vitro. Int. J. Med. Microbiol. 301, 204–212 - PubMed
-
- Brückener K. E., el Bayâ A., Galla H. J., Schmidt M. A. (2003) Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J. Cell Sci. 116, 1837–1846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

