Nuclear Calcium/Calmodulin-dependent Protein Kinase II Signaling Enhances Cardiac Progenitor Cell Survival and Cardiac Lineage Commitment
- PMID: 26324717
- PMCID: PMC4646189
- DOI: 10.1074/jbc.M115.657775
Nuclear Calcium/Calmodulin-dependent Protein Kinase II Signaling Enhances Cardiac Progenitor Cell Survival and Cardiac Lineage Commitment
Abstract
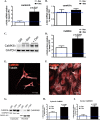
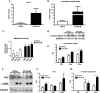
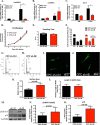
Ca(2+)/Calmodulin-dependent protein kinase II (CaMKII) signaling in the heart regulates cardiomyocyte contractility and growth in response to elevated intracellular Ca(2+). The δB isoform of CaMKII is the predominant nuclear splice variant in the adult heart and regulates cardiomyocyte hypertrophic gene expression by signaling to the histone deacetylase HDAC4. However, the role of CaMKIIδ in cardiac progenitor cells (CPCs) has not been previously explored. During post-natal growth endogenous CPCs display primarily cytosolic CaMKIIδ, which localizes to the nuclear compartment of CPCs after myocardial infarction injury. CPCs undergoing early differentiation in vitro increase levels of CaMKIIδB in the nuclear compartment where the kinase may contribute to the regulation of CPC commitment. CPCs modified with lentiviral-based constructs to overexpress CaMKIIδB (CPCeδB) have reduced proliferative rate compared with CPCs expressing eGFP alone (CPCe). Additionally, stable expression of CaMKIIδB promotes distinct morphological changes such as increased cell surface area and length of cells compared with CPCe. CPCeδB are resistant to oxidative stress induced by hydrogen peroxide (H2O2) relative to CPCe, whereas knockdown of CaMKIIδB resulted in an up-regulation of cell death and cellular senescence markers compared with scrambled treated controls. Dexamethasone (Dex) treatment increased mRNA and protein expression of cardiomyogenic markers cardiac troponin T and α-smooth muscle actin in CPCeδB compared with CPCe, suggesting increased differentiation. Therefore, CaMKIIδB may serve as a novel modulatory protein to enhance CPC survival and commitment into the cardiac and smooth muscle lineages.
Keywords: Ca2+/calmodulin-dependent protein kinase II (CaMKII); cell death; cell differentiation; histone deacetylase 4 (HDAC4); stem cells.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Rota M., Padin-Iruegas M. E., Misao Y., De Angelis A., Maestroni S., Ferreira-Martins J., Fiumana E., Rastaldo R., Arcarese M. L., Mitchell T. S., Boni A., Bolli R., Urbanek K., Hosoda T., Anversa P., Leri A., Kajstura J. (2008) Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ. Res. 103, 107–116 - PMC - PubMed
-
- Tallini Y. N., Greene K. S., Craven M., Spealman A., Breitbach M., Smith J., Fisher P. J., Steffey M., Hesse M., Doran R. M., Woods A., Singh B., Yen A., Fleischmann B. K., Kotlikoff M. I. (2009) c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc. Natl. Acad. Sci. U.S.A. 106, 1808–1813 - PMC - PubMed
-
- Ellison G. M., Vicinanza C., Smith A. J., Aquila I., Leone A., Waring C. D., Henning B. J., Stirparo G. G., Papait R., Scarfò M., Agosti V., Viglietto G., Condorelli G., Indolfi C., Ottolenghi S., Torella D., Nadal-Ginard B. (2013) Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL122525/HL/NHLBI NIH HHS/United States
- R01 HL105759/HL/NHLBI NIH HHS/United States
- P01 HL080101/HL/NHLBI NIH HHS/United States
- F31 HL117623/HL/NHLBI NIH HHS/United States
- P01HL085577/HL/NHLBI NIH HHS/United States
- R01HL067245/HL/NHLBI NIH HHS/United States
- R37 HL091102/HL/NHLBI NIH HHS/United States
- R37HL091102/HL/NHLBI NIH HHS/United States
- R01 HL113647/HL/NHLBI NIH HHS/United States
- R01 HL030077/HL/NHLBI NIH HHS/United States
- R01HL105759/HL/NHLBI NIH HHS/United States
- R01HL113647/HL/NHLBI NIH HHS/United States
- R01HL117163/HL/NHLBI NIH HHS/United States
- R01 HL067245/HL/NHLBI NIH HHS/United States
- P01 HL085577/HL/NHLBI NIH HHS/United States
- R01 HL117163/HL/NHLBI NIH HHS/United States
- R01HL122525/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

