Retinal Pigment Epithelial Cells Mitigate the Effects of Complement Attack by Endocytosis of C5b-9
- PMID: 26324770
- PMCID: PMC4574521
- DOI: 10.4049/jimmunol.1500937
Retinal Pigment Epithelial Cells Mitigate the Effects of Complement Attack by Endocytosis of C5b-9
Abstract
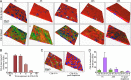
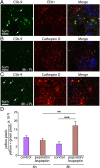
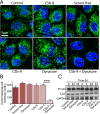
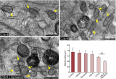
Retinal pigment epithelial (RPE) cell death is a hallmark of age-related macular degeneration. The alternative pathway of complement activation is strongly implicated in RPE cell dysfunction and loss in age-related macular degeneration; therefore, it is critical that RPE cells use molecular strategies to mitigate the potentially harmful effects of complement attack. We show that the terminal complement complex C5b-9 assembles rapidly on the basal surface of cultured primary porcine RPE cells but disappears over 48 h without any discernable adverse effects on the cells. However, in the presence of the dynamin inhibitor dynasore, C5b-9 was almost completely retained at the cell surface, suggesting that, under normal circumstances, it is eliminated via the endocytic pathway. In support of this idea, we observed that C5b-9 colocalizes with the early endosome marker EEA1 and that, in the presence of protease inhibitors, it can be detected in lysosomes. Preventing the endocytosis of C5b-9 by RPE cells led to structural defects in mitochondrial morphology consistent with cell stress. We conclude that RPE cells use the endocytic pathway to prevent the accumulation of C5b-9 on the cell surface and that processing and destruction of C5b-9 by this route are essential for RPE cell survival.
Copyright © 2015 The Authors.
Figures

References
-
- Mettu P. S., Wielgus A. R., Ong S. S., Cousins S. W. 2012. Retinal pigment epithelium response to oxidant injury in the pathogenesis of early age-related macular degeneration. Mol. Aspects Med. 33: 376–398. - PubMed
-
- Johnson L. V., Ozaki S., Staples M. K., Erickson P. A., Anderson D. H. 2000. A potential role for immune complex pathogenesis in drusen formation. Exp. Eye Res. 70: 441–449. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

