Ten Weeks of Infection with a Tissue-Invasive Helminth Protects against Local Immune Complex-Mediated Inflammation, but Not Cutaneous Type I Hypersensitivity, in Previously Sensitized Mice
- PMID: 26324775
- PMCID: PMC4651003
- DOI: 10.4049/jimmunol.1500081
Ten Weeks of Infection with a Tissue-Invasive Helminth Protects against Local Immune Complex-Mediated Inflammation, but Not Cutaneous Type I Hypersensitivity, in Previously Sensitized Mice
Abstract
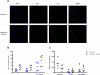
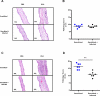
In this study, we evaluated the effect chronic helminth infection has on allergic disease in mice previously sensitized to OVA. Ten weeks of infection with Litomosoides sigmodontis reduced immunological markers of type I hypersensitivity, including OVA-specific IgE, basophil activation, and mast cell degranulation. Despite these reductions, there was no protection against immediate clinical hypersensitivity following intradermal OVA challenge. However, late-phase ear swelling, due to type III hypersensitivity, was significantly reduced in chronically infected animals. Levels of total IgG2a, OVA-specific IgG2a, and OVA-specific IgG1 were reduced in the setting of infection. These reductions were most likely due to increased Ab catabolism as ELISPOT assays demonstrated that infected animals do not have suppressed Ab production. Ear histology 24 h after challenge showed infected animals have reduced cellular infiltration in the ear, with significant decreases in numbers of neutrophils and macrophages. Consistent with this, infected animals had less neutrophil-specific chemokines CXCL-1 and CXCL-2 in the ear following challenge. Additionally, in vitro stimulation with immune complexes resulted in significantly less CXCL-1 and CXCL-2 production by eosinophils from chronically infected mice. Expression of FcγRI was also significantly reduced on eosinophils from infected animals. These data indicate that chronic filarial infection suppresses eosinophilic responses to Ab-mediated activation and has the potential to be used as a therapeutic for pre-existing hypersensitivity diseases.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures






References
-
- Bager P, Arnved J, Rønborg S, Wohlfahrt J, Poulsen LK, Westergaard T, Petersen HW, Kristensen B, Thamsborg S, Roepstorff A, Kapel C, Melbye M. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J. Allergy Clin. Immunol. 2010;125:123–130. - PubMed
-
- Evans H, Mitre E. Worms as therapeutic agents for allergy and asthma: Understanding why benefits in animal studies have not translated into clinical success. J. Allergy Clin. Immunol. 2015;135:343–353. - PubMed
-
- Hoffmann W, Petit G, Schulz-Key H, Taylor D, Bain O, Le Goff L. Litomosoides sigmodontis in mice: Reappraisal of an old model for filarial research. Parasitol. Today. 2000;16:387–389. - PubMed
-
- Pfaff AW, Schulz-Key H, Soboslay PT, Taylor DW, MacLennan K, Hoffmann WH. Litomosoides sigmodontis cystatin acts as an immunomodulator during experimental filariasis. Int. J. Parasitol. 2002;32:171–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

