Noncanonical role of transferrin receptor 1 is essential for intestinal homeostasis
- PMID: 26324903
- PMCID: PMC4577163
- DOI: 10.1073/pnas.1511701112
Noncanonical role of transferrin receptor 1 is essential for intestinal homeostasis
Abstract
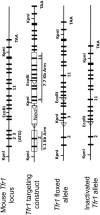
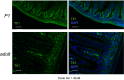
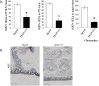
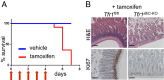
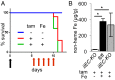
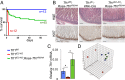
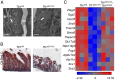
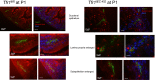
Transferrin receptor 1 (Tfr1) facilitates cellular iron uptake through receptor-mediated endocytosis of iron-loaded transferrin. It is expressed in the intestinal epithelium but not involved in dietary iron absorption. To investigate its role, we inactivated the Tfr1 gene selectively in murine intestinal epithelial cells. The mutant mice had severe disruption of the epithelial barrier and early death. There was impaired proliferation of intestinal epithelial cell progenitors, aberrant lipid handling, increased mRNA expression of stem cell markers, and striking induction of many genes associated with epithelial-to-mesenchymal transition. Administration of parenteral iron did not improve the phenotype. Surprisingly, however, enforced expression of a mutant allele of Tfr1 that is unable to serve as a receptor for iron-loaded transferrin appeared to fully rescue most animals. Our results implicate Tfr1 in homeostatic maintenance of the intestinal epithelium, acting through a role that is independent of its iron-uptake function.
Keywords: epithelial–mesenchymal transition; homeostasis; intestinal epithelium; stem cell; transferrin receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: Molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–297. - PubMed
-
- Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21(4):396–399. - PubMed
-
- Ned RM, Swat W, Andrews NC. Transferrin receptor 1 is differentially required in lymphocyte development. Blood. 2003;102(10):3711–3718. - PubMed
-
- Trenor CC, 3rd, Campagna DR, Sellers VM, Andrews NC, Fleming MD. The molecular defect in hypotransferrinemic mice. Blood. 2000;96(3):1113–1118. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

