The genome of Aiptasia, a sea anemone model for coral symbiosis
- PMID: 26324906
- PMCID: PMC4586855
- DOI: 10.1073/pnas.1513318112
The genome of Aiptasia, a sea anemone model for coral symbiosis
Abstract
The most diverse marine ecosystems, coral reefs, depend upon a functional symbiosis between a cnidarian animal host (the coral) and intracellular photosynthetic dinoflagellate algae. The molecular and cellular mechanisms underlying this endosymbiosis are not well understood, in part because of the difficulties of experimental work with corals. The small sea anemone Aiptasia provides a tractable laboratory model for investigating these mechanisms. Here we report on the assembly and analysis of the Aiptasia genome, which will provide a foundation for future studies and has revealed several features that may be key to understanding the evolution and function of the endosymbiosis. These features include genomic rearrangements and taxonomically restricted genes that may be functionally related to the symbiosis, aspects of host dependence on alga-derived nutrients, a novel and expanded cnidarian-specific family of putative pattern-recognition receptors that might be involved in the animal-algal interactions, and extensive lineage-specific horizontal gene transfer. Extensive integration of genes of prokaryotic origin, including genes for antimicrobial peptides, presumably reflects an intimate association of the animal-algal pair also with its prokaryotic microbiome.
Keywords: coral reefs; dinoflagellate; endosymbiosis; horizontal gene transfer; pattern-recognition receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

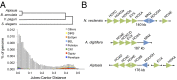

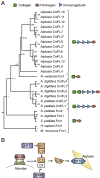
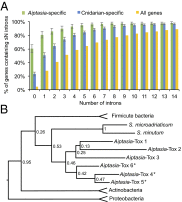
References
-
- Dubinsky Z, Stambler N. Coral Reefs: An Ecosystem in Transition. Springer Science & Business Media; Berlin: 2010.
-
- Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318(5857):1737–1742. - PubMed
-
- Weis VM, Davy SK, Hoegh-Guldberg O, Rodriguez-Lanetty M, Pringle JR. Cell biology in model systems as the key to understanding corals. Trends Ecol Evol. 2008;23(7):369–376. - PubMed
-
- Thornhill DJ, Xiang Y, Pettay DT, Zhong M, Santos SR. Population genetic data of a model symbiotic cnidarian system reveal remarkable symbiotic specificity and vectored introductions across ocean basins. Mol Ecol. 2013;22(17):4499–4515. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

