Marine and terrestrial herbivores display convergent chemical ecology despite 400 million years of independent evolution
- PMID: 26324909
- PMCID: PMC4593077
- DOI: 10.1073/pnas.1508133112
Marine and terrestrial herbivores display convergent chemical ecology despite 400 million years of independent evolution
Abstract
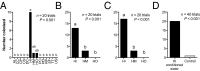
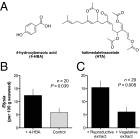
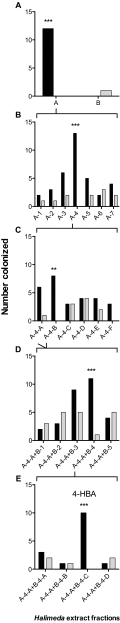
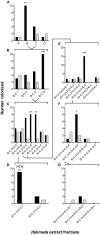
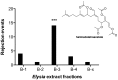
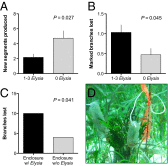
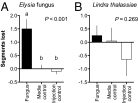
Chemical cues regulate key ecological interactions in marine and terrestrial ecosystems. They are particularly important in terrestrial plant-herbivore interactions, where they mediate both herbivore foraging and plant defense. Although well described for terrestrial interactions, the identity and ecological importance of herbivore foraging cues in marine ecosystems remain unknown. Here we show that the specialist gastropod Elysia tuca hunts its seaweed prey, Halimeda incrassata, by tracking 4-hydroxybenzoic acid to find vegetative prey and the defensive metabolite halimedatetraacetate to find reproductive prey. Foraging cues were predicted to be polar compounds but instead were nonpolar secondary metabolites similar to those used by specialist terrestrial insects. Tracking halimedatetraacetate enables Elysia to increase in abundance by 12- to 18-fold on reproductive Halimeda, despite reproduction in Halimeda being rare and lasting for only ∼36 h. Elysia swarm to reproductive Halimeda where they consume the alga's gametes, which are resource rich but are chemically defended from most consumers. Elysia sequester functional chloroplasts and halimedatetraacetate from Halimeda to become photosynthetic and chemically defended. Feeding by Elysia suppresses the growth of vegetative Halimeda by ∼50%. Halimeda responds by dropping branches occupied by Elysia, apparently to prevent fungal infection associated with Elysia feeding. Elysia is remarkably similar to some terrestrial insects, not only in its hunting strategy, but also its feeding method, defense tactics, and effects on prey behavior and performance. Such striking parallels indicate that specialist herbivores in marine and terrestrial systems can evolve convergent ecological strategies despite 400 million years of independent evolution in vastly different habitats.
Keywords: chemical cue; defense; eavesdropping; herbivory; prey tracking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3(7):408–414. - PubMed
-
- Karban R, Baldwin IT. Induced Responses to Herbivory. Univ of Chicago Press; Chicago: 1997.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

