Mesenchymal Stem Cell-Like Properties of Orbital Fibroblasts in Graves' Orbitopathy
- PMID: 26325413
- PMCID: PMC4559215
- DOI: 10.1167/iovs.15-16580
Mesenchymal Stem Cell-Like Properties of Orbital Fibroblasts in Graves' Orbitopathy
Abstract
Purpose: Graves' orbitopathy (GO) is a sight-threatening autoimmune disorder causing extraocular muscle fibrosis, upper lid retraction and eye bulging due to orbital fat expansion. These clinical features are mediated by aspects of orbital fibroblasts differentiation, including adipogenesis and fibrosis. Our previous work suggested that this dual phenotype might be a manifestation of mixed cell populations, partially linked to the expression of mesenchymal stem cell (MSC) marker CD90. Thus, we set out to determine whether GO orbital fibroblasts displayed MSC properties.
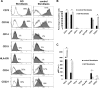
Methods: Control and GO orbital fibroblasts previously characterized for CD90 and CD45 expression were analyzed by flow cytometry for classical MSC positive (CD73, CD105) and negative (CD14, CD19, HLA-DR, and CD34) markers. Graves' orbitopathy fibroblasts were tested further for their ability to undergo lineage specific differentiation following standard protocols.
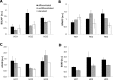
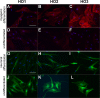
Results: Control and GO fibroblasts strongly expressed CD73 and CD105, with a higher percentage of positive cells and stronger expression levels in GO. Neither cell type expresses CD14, CD19, and HLA-DR. Protein CD34 was expressed at low levels by 45% to 70% of the cells, with its expression significantly lower in GO cells. Graves' orbitopathy fibroblasts displayed features of osteogenesis (calcium deposits, and osteocalcin [BGLAP] and osteonectin [SPARC] expression), chondrogenesis (glycosaminoglycan production; SOX9 and aggrecan [ACAN] expression), myogenesis (α-smooth muscle actin expression), and neurogenesis (β-III tubulin expression) upon differentiation.
Conclusions: Our findings suggest that orbital fibroblasts contain a population of cells that fulfil the criteria defining MSC. This subpopulation may be increased in GO, possibly underlying the complex differentiation phenotype of the disease.
Figures


Similar articles
-
Orbital Fibroblasts From Graves' Orbitopathy Patients Share Functional and Immunophenotypic Properties With Mesenchymal Stem/Stromal Cells.Invest Ophthalmol Vis Sci. 2015 Oct;56(11):6549-57. doi: 10.1167/iovs.15-16610. Invest Ophthalmol Vis Sci. 2015. PMID: 26457540
-
Isolation and Characterization of Extraocular Muscle-Derived Muscle Progenitor Cells from Normal and Graves' Orbitopathy Patients.Stem Cells Dev. 2020 Mar 15;29(6):353-363. doi: 10.1089/scd.2019.0212. Epub 2020 Feb 27. Stem Cells Dev. 2020. PMID: 31969085
-
Characterization of mesenchymal stem cell subpopulations from human amniotic membrane with dissimilar osteoblastic potential.Stem Cells Dev. 2013 Apr 15;22(8):1275-87. doi: 10.1089/scd.2012.0359. Epub 2013 Jan 29. Stem Cells Dev. 2013. PMID: 23211052
-
Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves' ophthalmopathy.Exp Eye Res. 2016 Jan;142:83-91. doi: 10.1016/j.exer.2015.02.007. Exp Eye Res. 2016. PMID: 26675405 Review.
-
Molecular biomarkers of Graves' ophthalmopathy.Exp Mol Pathol. 2019 Feb;106:1-6. doi: 10.1016/j.yexmp.2018.11.004. Epub 2018 Nov 8. Exp Mol Pathol. 2019. PMID: 30414981 Free PMC article. Review.
Cited by
-
The Effect of Prostaglandin Analogue Bimatoprost on Thyroid-Associated Orbitopathy.Invest Ophthalmol Vis Sci. 2018 Dec 3;59(15):5912-5923. doi: 10.1167/iovs.18-25134. Invest Ophthalmol Vis Sci. 2018. PMID: 30551199 Free PMC article.
-
New insights into the pathogenesis and nonsurgical management of Graves orbitopathy.Nat Rev Endocrinol. 2020 Feb;16(2):104-116. doi: 10.1038/s41574-019-0305-4. Epub 2019 Dec 30. Nat Rev Endocrinol. 2020. PMID: 31889140 Review.
-
A review of TSHR- and IGF-1R-related pathogenesis and treatment of Graves' orbitopathy.Front Immunol. 2023 Jan 19;14:1062045. doi: 10.3389/fimmu.2023.1062045. eCollection 2023. Front Immunol. 2023. PMID: 36742308 Free PMC article. Review.
-
Effect of Isolation Technique and Location on the Phenotype of Human Corneal Stroma-Derived Cells.Stem Cells Int. 2017;2017:9275248. doi: 10.1155/2017/9275248. Epub 2017 Oct 29. Stem Cells Int. 2017. PMID: 29213290 Free PMC article.
-
Paracrine signals of mesenchymal stem cells induce epithelial to mesenchymal transition in gastric cancer cells.Gastroenterol Hepatol Bed Bench. 2019;12(Suppl1):S51-S57. Gastroenterol Hepatol Bed Bench. 2019. PMID: 32099602 Free PMC article.
References
-
- Trobe JD. Optic nerve involvement in dysthyroidism. Ophthalmology. 1981; 88: 488–492. - PubMed
-
- Tallstedt L,, Lundell G,, Torring O,, et al. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. The Thyroid Study Group. N Engl J Med. 1992; 326: 1733–1738. - PubMed
-
- El-Kaissi S,, Frauman AG,, Wall JR. Thyroid-associated ophthalmopathy: a practical guide to classification, natural history and management. Intern Med J. 2004; 34: 482–491. - PubMed
-
- Regensburg NI,, Wiersinga WM,, Berendschot TT,, Potgieser P,, Mourits MP. Do subtypes of graves' orbitopathy exist? Ophthalmology. 2011; 118: 191–196. - PubMed
-
- Eckstein AK,, Johnson KT,, Thanos M,, Esser J,, Ludgate M. Current insights into the pathogenesis of Graves' orbitopathy. Horm Metab Res. 2009; 41: 456–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

