Cathepsin X Cleaves Profilin 1 C-Terminal Tyr139 and Influences Clathrin-Mediated Endocytosis
- PMID: 26325675
- PMCID: PMC4567178
- DOI: 10.1371/journal.pone.0137217
Cathepsin X Cleaves Profilin 1 C-Terminal Tyr139 and Influences Clathrin-Mediated Endocytosis
Abstract
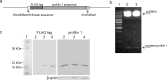
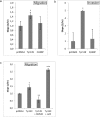
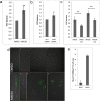
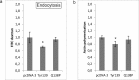
Cathepsin X, a cysteine carboxypeptidase, is upregulated in several types of cancer. Its molecular target in tumor cells is profilin 1, a known tumor suppressor and regulator of actin cytoskeleton dynamics. Cathepsin X cleaves off the C-terminal Tyr139 of profilin 1, affecting binding of poly-L-proline ligands and, consequently, tumor cell migration and invasion. Profilin 1 with mutations at the C-terminus, transiently expressed in prostate cancer cells PC-3, showed that Tyr139 is important for proper function of profilin 1 as a tumor suppressor. Cleaving off Tyr139 prevents the binding of clathrin, a poly-L-proline ligand involved in endocytosis. More profilin 1-clathrin complexes were present in PC-3 cells when cathepsin X was inhibited by its specific inhibitor AMS36 or silenced by siRNA. As a consequence, the endocytosis of FITC-labeled dextran and transferrin conjugate was significantly increased. These results constitute the first report of the regulation of clathrin-mediated endocytosis in tumor cells through proteolytic processing of profilin 1.
Conflict of interest statement
Figures


Similar articles
-
Profilin 1 as a target for cathepsin X activity in tumor cells.PLoS One. 2013;8(1):e53918. doi: 10.1371/journal.pone.0053918. Epub 2013 Jan 10. PLoS One. 2013. PMID: 23326535 Free PMC article.
-
Mouse profilin 2 regulates endocytosis and competes with SH3 ligand binding to dynamin 1.J Biol Chem. 2006 Feb 3;281(5):2803-11. doi: 10.1074/jbc.M503528200. Epub 2005 Nov 29. J Biol Chem. 2006. PMID: 16319076
-
High-resolution structural analysis of mammalian profilin 2a complex formation with two physiological ligands: the formin homology 1 domain of mDia1 and the proline-rich domain of VASP.J Mol Biol. 2008 Jan 4;375(1):270-90. doi: 10.1016/j.jmb.2007.10.050. Epub 2007 Oct 24. J Mol Biol. 2008. PMID: 18001770
-
[Profilins in plant cells].Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2006 Jun;32(3):261-70. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2006. PMID: 16775392 Review. Chinese.
-
Profilin, a multi-modal regulator of neuronal plasticity.Bioessays. 2008 Oct;30(10):994-1002. doi: 10.1002/bies.20822. Bioessays. 2008. PMID: 18798527 Review.
Cited by
-
Cofilin and profilin: partners in cancer aggressiveness.Biophys Rev. 2018 Oct;10(5):1323-1335. doi: 10.1007/s12551-018-0445-0. Epub 2018 Jul 19. Biophys Rev. 2018. PMID: 30027463 Free PMC article. Review.
-
Cathepsins in the Pathophysiology of Mucopolysaccharidoses: New Perspectives for Therapy.Cells. 2020 Apr 15;9(4):979. doi: 10.3390/cells9040979. Cells. 2020. PMID: 32326609 Free PMC article. Review.
-
Sorcin induces gastric cancer cell migration and invasion contributing to STAT3 activation.Oncotarget. 2017 Oct 31;8(61):104258-104271. doi: 10.18632/oncotarget.22208. eCollection 2017 Nov 28. Oncotarget. 2017. PMID: 29262638 Free PMC article.
-
Identification and characterization of the novel reversible and selective cathepsin X inhibitors.Sci Rep. 2017 Sep 13;7(1):11459. doi: 10.1038/s41598-017-11935-1. Sci Rep. 2017. PMID: 28904354 Free PMC article.
-
PPP1R14B as a potential biomarker for the identification of diagnosis and prognosis affecting tumor immunity, proliferation and migration in prostate cancer.J Cancer. 2024 Oct 21;15(20):6545-6564. doi: 10.7150/jca.101100. eCollection 2024. J Cancer. 2024. PMID: 39668827 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

