A Newly Identified Passive Hyperaccumulator Eucalyptus grandis × E. urophylla under Manganese Stress
- PMID: 26327118
- PMCID: PMC4556624
- DOI: 10.1371/journal.pone.0136606
A Newly Identified Passive Hyperaccumulator Eucalyptus grandis × E. urophylla under Manganese Stress
Abstract
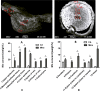
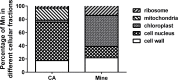
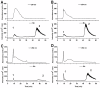
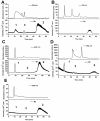
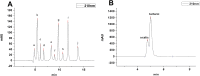
Manganese (Mn) is an essential micronutrient needed for plant growth and development, but can be toxic to plants in excess amounts. However, some plant species have detoxification mechanisms that allow them to accumulate Mn to levels that are normally toxic, a phenomenon known as hyperaccumulation. These species are excellent candidates for developing a cost-effective remediation strategy for Mn-polluted soils. In this study, we identified a new passive Mn-hyperaccumulator Eucalyptus grandis × E. urophylla during a field survey in southern China in July 2010. This hybrid can accumulate as much as 13,549 mg/kg DW Mn in its leaves. Our results from Scanning Electron Microscope (SEM) X-ray microanalysis indicate that Mn is distributed in the entire leaf and stem cross-section, especially in photosynthetic palisade, spongy mesophyll tissue, and stem xylem vessels. Results from size-exclusion chromatography coupled with ICP-MS (Inductively coupled plasma mass spectrometry) lead us to speculate that Mn associates with relatively high molecular weight proteins and low molecular weight organic acids, including tartaric acid, to avoid Mn toxicity. Our results provide experimental evidence that both proteins and organic acids play important roles in Mn detoxification in Eucalyptus grandis × E. urophylla. The key characteristics of Eucalyptus grandis × E. urophylla are an increased Mn translocation facilitated by transpiration through the xylem to the leaves and further distribution throughout the leaf tissues. Moreover, the Mn-speciation profile obtained for the first time in different cellular organelles of Eucalyptus grandis × E. urophylla suggested that different organelles have differential accumulating abilities and unique mechanisms for Mn-detoxification.
Conflict of interest statement
Figures


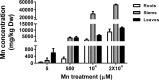
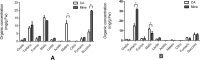
References
-
- Horst M. Mineral nutrition of higher plants Academic Press; 2011.
-
- Karki P LE, Aschner M. Manganese neurotoxicity: a focus on glutamate transporters. Annals of occupational and environmental medicine. 2013;25(4). Available: http://www.aoemj.com/content/25/1/4. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

