Membrane associated cancer-oocyte neoantigen SAS1B/ovastacin is a candidate immunotherapeutic target for uterine tumors
- PMID: 26327203
- PMCID: PMC4745790
- DOI: 10.18632/oncotarget.4734
Membrane associated cancer-oocyte neoantigen SAS1B/ovastacin is a candidate immunotherapeutic target for uterine tumors
Erratum in
-
Correction: Membrane associated cancer-oocyte neoantigen SAS1B/ovastacin is a candidate immunotherapeutic target for uterine tumors.Oncotarget. 2017 Feb 28;8(9):16099. doi: 10.18632/oncotarget.15755. Oncotarget. 2017. PMID: 28403580 Free PMC article. No abstract available.
Abstract
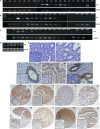
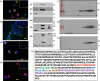
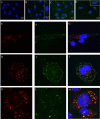
The metalloproteinase SAS1B [ovastacin, ASTL, astacin-like] was immunolocalized on the oolemma of ovulated human oocytes and in normal ovaries within the pool of growing oocytes where SAS1B protein was restricted to follicular stages spanning the primary-secondary follicle transition through ovulation. Gene-specific PCR and immunohistochemical studies revealed ASTL messages and SAS1B protein in both endometrioid [74%] and malignant mixed Mullerian tumors (MMMT) [87%] of the uterus. A MMMT-derived cell line, SNU539, expressed cell surface SAS1B that, after binding polyclonal antibodies, internalized into EEA1/LAMP1-positive early and late endosomes. Treatment of SNU539 cells with anti-SAS1B polyclonal antibodies caused growth arrest in the presence of active complement. A saporin-immunotoxin directed to SAS1B induced growth arrest and cell death. The oocyte restricted expression pattern of SAS1B among adult organs, cell-surface accessibility, internalization into the endocytic pathway, and tumor cell growth arrest induced by antibody-toxin conjugates suggest therapeutic approaches that would selectively target tumors while limiting adverse drug effects in healthy cells. The SAS1B metalloproteinase is proposed as a prototype cancer-oocyte tumor surface neoantigen for development of targeted immunotherapeutics with limited on-target/off tumor effects predicted to be restricted to the population of growing oocytes.
Keywords: ASTL/SAS1B/ovastacin; cancer surface neoantigen; immunotherapy target; oocyte-specific protein; uterine tumor biomarkers.
Conflict of interest statement
The University of Virginia has filed patent applications on the use of the SAS1B as a cancer drug and diagnostic target. Neoantigenics has an exclusive license. E.P, J.H., A.M., K.A., and B.P. hold equity in Neoantigenics.
Figures




References
-
- Sachdev M, Mandal A, Mulders S, Digilio LC, Panneerdoss S, Suryavathi V, Pires E, Klotz KL, Hermens L, Herrero MB, Flickinger CJ, van Duin M, Herr JC. Oocyte specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization. Developmental Biology. 2012;363:40–51. - PMC - PubMed
-
- Pires ES, Hlavin C, Macnamara E, Ishola-Gbenla K, Doerwaldt C, Chamberlain C, Klotz K, Herr AK, Khole A, Chertihin O, Curnow E, Feldman SH, Mandal A, et al. SAS1B protein [Ovastacin] shows temporal and spatial restriction to oocytes in several eutherian orders and initiates translation at the primary to secondary follicle transition. Developmental Dynamics. 2013;242:1405–26. - PMC - PubMed
-
- Mouse EST dataset from GENBANK for Astl: http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Mm.31313.
-
- Website: human ASTL EST database, http://www.ncbi.nlm.nih.gov/nucest/14468989 and http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.447993.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

