Drug Development in Conformational Diseases: A Novel Family of Chemical Chaperones that Bind and Stabilise Several Polymorphic Amyloid Structures
- PMID: 26327208
- PMCID: PMC4556714
- DOI: 10.1371/journal.pone.0135292
Drug Development in Conformational Diseases: A Novel Family of Chemical Chaperones that Bind and Stabilise Several Polymorphic Amyloid Structures
Abstract
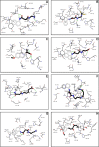
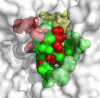
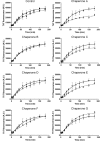
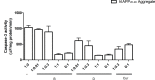
The increasing prevalence of conformational diseases, including Alzheimer's disease, type 2 Diabetes Mellitus and Cancer, poses a global challenge at many different levels. It has devastating effects on the sufferers as well as a tremendous economic impact on families and the health system. In this work, we apply a cross-functional approach that combines ideas, concepts and technologies from several disciplines in order to study, in silico and in vitro, the role of a novel chemical chaperones family (NCHCHF) in processes of protein aggregation in conformational diseases. Given that Serum Albumin (SA) is the most abundant protein in the blood of mammals, and Bovine Serum Albumin (BSA) is an off-the-shelf protein available in most labs around the world, we compared the ligandability of BSA:NCHCHF with the interaction sites in the Human Islet Amyloid Polypeptide (hIAPP):NCHCHF, and in the amyloid pharmacophore fragments (Aβ17-42 and Aβ16-21):NCHCHF. We posit that the merging of this interaction sites is a meta-structure of pharmacophore which allows the development of chaperones that can prevent protein aggregation at various states from: stabilizing the native state to destabilizing oligomeric state and protofilament. Furthermore to stabilize fibrillar structures, thus decreasing the amount of toxic oligomers in solution, as is the case with the NCHCHF. The paper demonstrates how a set of NCHCHF can be used for studying and potentially treating the various physiopathological stages of a conformational disease. For instance, when dealing with an acute phase of cytotoxicity, what is needed is the recruitment of cytotoxic oligomers, thus chaperone F, which accelerates fiber formation, would be very useful; whereas in a chronic stage it is better to have chaperones A, B, C, and D, which stabilize the native and fibril structures halting self-catalysis and the creation of cytotoxic oligomers as a consequence of fiber formation. Furthermore, all the chaperones are able to protect and recondition the cerebellar granule cells (CGC) from the cytotoxicity produced by the hIAPP20-29 fragment or by a low potassium medium, regardless of their capacity for accelerating or inhibiting in vitro formation of fibers. In vivo animal experiments are required to study the impact of chemical chaperones in cognitive and metabolic syndromes.
Conflict of interest statement
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

