Vector-Host Interactions of Culiseta melanura in a Focus of Eastern Equine Encephalitis Virus Activity in Southeastern Virginia
- PMID: 26327226
- PMCID: PMC4556703
- DOI: 10.1371/journal.pone.0136743
Vector-Host Interactions of Culiseta melanura in a Focus of Eastern Equine Encephalitis Virus Activity in Southeastern Virginia
Abstract
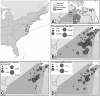
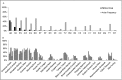
Eastern equine encephalitis virus (EEEV) causes a highly pathogenic mosquito-borne zoonosis that is responsible for sporadic outbreaks of severe illness in humans and equines in the eastern USA. Culiseta (Cs.) melanura is the primary vector of EEEV in most geographic regions but its feeding patterns on specific avian and mammalian hosts are largely unknown in the mid-Atlantic region. The objectives of our study were to: 1) identify avian hosts of Cs. melanura and evaluate their potential role in enzootic amplification of EEEV, 2) assess spatial and temporal patterns of virus activity during a season of intense virus transmission, and 3) investigate the potential role of Cs. melanura in epidemic/epizootic transmission of EEEV to humans and equines. Accordingly, we collected mosquitoes at 55 sites in Suffolk, Virginia in 2013, and identified the source of blood meals in engorged mosquitoes by nucleotide sequencing PCR products of the mitochondrial cytochrome b gene. We also examined field-collected mosquitoes for evidence of infection with EEEV using Vector Test, cell culture, and PCR. Analysis of 188 engorged Cs. melanura sampled from April through October 2013 indicated that 95.2%, 4.3%, and 0.5% obtained blood meals from avian, mammalian, and reptilian hosts, respectively. American Robin was the most frequently identified host for Cs. melanura (42.6% of blood meals) followed by Northern Cardinal (16.0%), European Starling (11.2%), Carolina Wren (4.3%), and Common Grackle (4.3%). EEEV was detected in 106 mosquito pools of Cs. melanura, and the number of virus positive pools peaked in late July with 22 positive pools and a Maximum Likelihood Estimation (MLE) infection rate of 4.46 per 1,000 mosquitoes. Our findings highlight the importance of Cs. melanura as a regional EEEV vector based on frequent feeding on virus-competent bird species. A small proportion of blood meals acquired from mammalian hosts suggests the possibility that this species may occasionally contribute to epidemic/epizootic transmission of EEEV.
Conflict of interest statement
Figures

References
-
- Morris CD. Eastern equine encephalomyelitis Monath TP, ed. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: 1988. CRC Press, 1–20.
-
- Scott TW, Weaver SC. Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res. 1989; 37: 277–328. - PubMed
-
- Edman JD. Host-feeding patterns of Florida mosquitoes I. Aedes, Anopheles, Coquillettidia, Mansonia, and Psorophora. J Med Entomol. 1971; 8: 687–95. - PubMed
-
- Nasci RS, Edman JD. Blood-feeding patterns of Culiseta melanura (Diptera: Culicidae) and associated sylvan mosquitoes in southeastern Massachusetts eastern equine encephalitis enzootic foci. J Med Entomol. 1981; 18: 493–500.
-
- Molaei G, Andreadis TG. Identification of avian- and mammalian-derived blood meals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, USA. J Med Entomol. 2006; 43: 1088–1093. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

