Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: a meta-analysis
- PMID: 26327302
- PMCID: PMC4844196
- DOI: 10.1080/15592294.2015.1088630
Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: a meta-analysis
Abstract
Prenatal stress has been widely associated with a number of short- and long-term pathological outcomes. Epigenetic mechanisms are thought to partially mediate these environmental insults into the fetal physiology. One of the main targets of developmental programming is the hypothalamic-pituitary-adrenal (HPA) axis as it is the main regulator of the stress response. Accordingly, an increasing number of researchers have recently focused on the putative association between DNA methylation at the glucocorticoid receptor gene (NR3C1) and prenatal stress, among other types of psychosocial stress. The current study aims to systematically review and meta-analyze the existing evidence linking several forms of prenatal stress with DNA methylation at the region 1F of the NR3C1 gene. The inclusion of relevant articles allowed combining empirical evidence from 977 individuals by meta-analytic techniques, whose methylation assessments showed overlap across 5 consecutive CpG sites (GRCh37/hg19 chr5:142,783,607-142,783,639). From this information, methylation levels at CpG site 36 displayed a significant correlation to prenatal stress (r = 0.14, 95% CI: 0.05-0.23, P = 0.002). This result supports the proposed association between a specific CpG site located at the NR3C1 promoter and prenatal stress. Several confounders, such as gender, methylation at other glucocorticoid-related genes, and adjustment for pharmacological treatments during pregnancy, should be taken into account in further studies.
Keywords: DNA methylation; HPA axis; NR3C1 gene; epigenetics; glucocorticoid receptor; maternal stress.
Figures
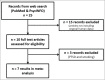
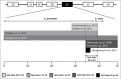

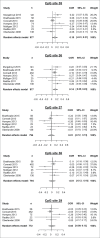
Similar articles
-
Glucocorticoid receptor gene methylation moderates the association of childhood trauma and cortisol stress reactivity.Psychoneuroendocrinology. 2018 Apr;90:68-75. doi: 10.1016/j.psyneuen.2018.01.020. Epub 2018 Jan 31. Psychoneuroendocrinology. 2018. PMID: 29433075
-
Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses.Epigenetics. 2008 Mar-Apr;3(2):97-106. doi: 10.4161/epi.3.2.6034. Epigenetics. 2008. PMID: 18536531
-
Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review.Neurosci Biobehav Rev. 2015 Aug;55:520-35. doi: 10.1016/j.neubiorev.2015.05.016. Epub 2015 Jun 11. Neurosci Biobehav Rev. 2015. PMID: 26073068 Review.
-
Maternal perceived stress and green spaces during pregnancy are associated with adult offspring gene (NR3C1 and IGF2/H19) methylation patterns in adulthood: A pilot study.Psychoneuroendocrinology. 2024 Sep;167:107088. doi: 10.1016/j.psyneuen.2024.107088. Epub 2024 May 31. Psychoneuroendocrinology. 2024. PMID: 38924829
-
Glucocorticoid receptor gene (NR3C1) DNA methylation in association with trauma, psychopathology, transcript expression, or genotypic variation: A systematic review.Neurosci Biobehav Rev. 2018 Dec;95:85-122. doi: 10.1016/j.neubiorev.2018.08.017. Epub 2018 Aug 31. Neurosci Biobehav Rev. 2018. PMID: 30176278
Cited by
-
Cumulative lifetime maternal stress and epigenome-wide placental DNA methylation in the PRISM cohort.Epigenetics. 2018;13(6):665-681. doi: 10.1080/15592294.2018.1497387. Epub 2018 Aug 15. Epigenetics. 2018. PMID: 30001177 Free PMC article.
-
Food Addiction and Psychosocial Adversity: Biological Embedding, Contextual Factors, and Public Health Implications.Nutrients. 2020 Nov 16;12(11):3521. doi: 10.3390/nu12113521. Nutrients. 2020. PMID: 33207612 Free PMC article. Review.
-
Epigenetics: A Missing Link Between Early Life Stress and Depression.Adv Exp Med Biol. 2021;1305:117-128. doi: 10.1007/978-981-33-6044-0_8. Adv Exp Med Biol. 2021. PMID: 33834398 Review.
-
GR/Ahi1 regulates WDR68-DYRK1A binding and mediates cognitive impairment in prenatally stressed offspring.Cell Mol Life Sci. 2024 Jan 10;81(1):20. doi: 10.1007/s00018-023-05075-1. Cell Mol Life Sci. 2024. PMID: 38195774 Free PMC article.
-
Maternal Lifetime Trauma and Birthweight: Effect Modification by In Utero Cortisol and Child Sex.J Pediatr. 2018 Dec;203:301-308. doi: 10.1016/j.jpeds.2018.07.069. Epub 2018 Sep 6. J Pediatr. 2018. PMID: 30197200 Free PMC article.
References
-
- Kofink D, Boks MPM, Timmers HTM, Kas MJ. Epigenetic dynamics in psychiatric disorders: environmental programming of neurodevelopmental processes. Neurosci Biobehav Rev 2013; 37:831-45; PMID:23567520; http://dx.doi.org/ 10.1016/j.neubiorev.2013.03.020 - DOI - PubMed
-
- Naumova OY, Lee M, Rychkov SY, Vlasova NV, Grigorenko EL. Gene expression in the human brain: the current state of the study of specificity and spatiotemporal dynamics. Child Dev 2013; 84:76-88; PMID:23145569; http://dx.doi.org/ 10.1111/cdev.12014 - DOI - PMC - PubMed
-
- Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, Colantuoni EA, Elkahloun AG, Herman MM, Weinberger DR, et al.. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature 2011; 478:519-23; PMID:22031444; http://dx.doi.org/ 10.1038/nature10524 - DOI - PMC - PubMed
-
- Hanson M a, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 2014; 94:1027-76; PMID:25287859; http://dx.doi.org/ 10.1152/physrev.00029.2013 - DOI - PMC - PubMed
-
- Delisle H. Programming of chronic disease by impaired fetal nutrition. Evidence and implications for policy and intervention strategies. World Health Organization 2001; Retrieved from: http://www.who.int/nutrition/publications/obesity/WHO_NHD_02.3/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
