Hybrid Theranostic Platform for Second Near-IR Window Light Triggered Selective Two-Photon Imaging and Photothermal Killing of Targeted Melanoma Cells
- PMID: 26327304
- PMCID: PMC4669052
- DOI: 10.1021/acsami.5b05225
Hybrid Theranostic Platform for Second Near-IR Window Light Triggered Selective Two-Photon Imaging and Photothermal Killing of Targeted Melanoma Cells
Abstract
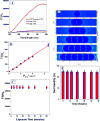
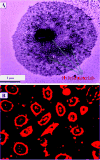
Despite advances in the medical field, even in the 21st century cancer is one of the leading causes of death for men and women in the world. Since the second near-infrared (NIR) biological window light between 950 and 1350 nm offers highly efficient tissue penetration, the current article reports the development of hybrid theranostic platform using anti-GD2 antibody attached gold nanoparticle (GNP) conjugated, single-wall carbon nanotube (SWCNT) for second near-IR light triggered selective imaging and efficient photothermal therapy of human melanoma cancer cell. Reported results demonstrate that due to strong plasmon-coupling, two-photon luminescence (TPL) intensity from theranostic GNP attached SWCNT materials is 6 orders of magnitude higher than GNP or SWCNT alone. Experimental and FDTD simulation data indicate that the huge enhancement of TPL intensity is mainly due to strong resonance enhancement coupled with the stronger electric field enhancement. Due to plasmon coupling, the theranostic material serves as a local nanoantennae to enhance the photothermal capability via strong optical energy absorption. Reported data show that theranostic SWCNT can be used for selective two-photon imaging of melanoma UACC903 cell using 1100 nm light. Photothermal killing experiment with 1.0 W/cm(2) 980 nm laser light demonstrates that 100% of melanoma UACC903 cells can be killed using theranostic SWCNT bind melanoma cells after just 8 min of exposure. These results demonstrate that due to plasmon coupling, the theranostic GNP attached SWCNT material serves as a two-photon imaging and photothermal source for cancer cells in biological window II.
Keywords: FDTD simulation; hybrid plasmonic CNT; second biological window; selective photothermal therapy; theranostic platform; two-photon imaging of human melanoma cancer cell.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Theranostic nanobubble encapsulating a plasmon-enhanced upconversion hybrid nanosystem for cancer therapy.Theranostics. 2020 Jan 1;10(2):782-796. doi: 10.7150/thno.38684. eCollection 2020. Theranostics. 2020. PMID: 31903150 Free PMC article.
-
Antibody-Conjugated Gel-Coated Single-Walled Carbon Nanotubes as Photothermal Agents.ACS Appl Bio Mater. 2021 Jun 21;4(6):5049-5056. doi: 10.1021/acsabm.1c00299. Epub 2021 May 26. ACS Appl Bio Mater. 2021. PMID: 35007053
-
Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications.J Nanobiotechnology. 2018 Oct 13;16(1):80. doi: 10.1186/s12951-018-0405-7. J Nanobiotechnology. 2018. PMID: 30316298 Free PMC article.
-
Second Near-Infrared Plasmonic Nanomaterials for Photoacoustic Imaging and Photothermal Therapy.Small. 2023 Jul;19(30):e2300539. doi: 10.1002/smll.202300539. Epub 2023 Apr 14. Small. 2023. PMID: 37060228 Review.
-
Theranostics of Gold Nanoparticles with an Emphasis on Photoacoustic Imaging and Photothermal Therapy.Curr Pharm Des. 2018;24(23):2719-2728. doi: 10.2174/1381612824666180604112201. Curr Pharm Des. 2018. PMID: 29865999 Review.
Cited by
-
Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017 Jul;9(4):10.1002/wnan.1449. doi: 10.1002/wnan.1449. Epub 2017 Feb 3. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017. PMID: 28160445 Free PMC article. Review.
-
Cu-Hemin Nanosheets and Indocyanine Green Co-Loaded Hydrogel for Photothermal Therapy and Amplified Photodynamic Therapy.Front Oncol. 2022 Jun 30;12:918416. doi: 10.3389/fonc.2022.918416. eCollection 2022. Front Oncol. 2022. PMID: 35847901 Free PMC article.
-
Radiopaque Crystalline, Non-Crystalline and Nanostructured Bioceramics.Materials (Basel). 2022 Oct 25;15(21):7477. doi: 10.3390/ma15217477. Materials (Basel). 2022. PMID: 36363085 Free PMC article. Review.
-
A bibliometric analysis and visualization of photothermal therapy on cancer.Transl Cancer Res. 2021 Mar;10(3):1204-1215. doi: 10.21037/tcr-20-2961. Transl Cancer Res. 2021. PMID: 35116448 Free PMC article.
-
Synergistic effect of sunlight induced photothermal conversion and H2O2 release based on hybridized tungsten oxide gel for cancer inhibition.Sci Rep. 2016 Oct 24;6:35876. doi: 10.1038/srep35876. Sci Rep. 2016. PMID: 27775086 Free PMC article.
References
-
- Van Denderen BJE, Thompson EW. Cancer: The to and fro of Tumour Spread. Nature. 2013;493:487–488. - PubMed
-
- Lim EK, Kim T, Paik S, Haam S, Huh YM, Lee K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem Rev. 2015;115:327–39. - PubMed
-
- Demeritte T, Fan Z, Sinha SS, Duan J, Pachter R, Ray PC. Gold Nanocage Assembly for Selective Second Harmonic Generation Imaging of Cancer Cell. Chem—Eur J. 2014;20:1017–1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

