PAQR3 regulates Golgi vesicle fission and transport via the Gβγ-PKD signaling pathway
- PMID: 26327583
- PMCID: PMC4684484
- DOI: 10.1016/j.cellsig.2015.08.017
PAQR3 regulates Golgi vesicle fission and transport via the Gβγ-PKD signaling pathway
Abstract
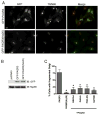
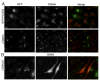
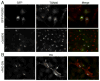
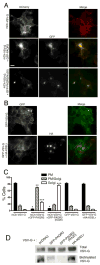
Heterotrimeric G proteins function at diverse subcellular locations, in addition to canonical signaling at the plasma membrane (PM). Gβγ signals at the Golgi, via protein kinase D (PKD), to regulate fission of PM-destined vesicles. However, the mechanism by which Gβγ is regulated at the Golgi in this process remains elusive. Recent studies have revealed that PAQR3 (Progestin and AdipoQ Receptor 3), also called RKTG (Raf Kinase Trapping to the Golgi), interacts with the Gβ subunit and localizes Gβ to the Golgi thereby inhibiting Gβγ signaling at the PM. Herein we show that, in contrast to this inhibition of canonical Gβγ signaling at the PM, PAQR3 promotes Gβγ signaling at the Golgi. Expression of PAQR3 causes fragmentation of the Golgi, while a Gβ binding-deficient mutant of PAQR3 does not cause Golgi fragmentation. Also, a C-terminal fragment of GRK2 (GRK2ct), which interacts with Gβγ and inhibits Gβγ signaling, and gallein, a small molecule inhibitor of Gβγ, are both able to inhibit PAQR3-mediated Golgi fragmentation. Furthermore, a dominant negative form of PKD (PKD-DN) and a pharmacological inhibitor of PKD, Gö6976, also inhibit PAQR3-mediated fragmentation of the Golgi. Importantly, expression of the Gβ binding-deficient mutant of PAQR3 inhibits the constitutive transport of the model cargo protein VSV-G from the Golgi to the PM, indicating the involvement of PAQR3 in Golgi-to PM vesicle transport and a dominant negative role for this mutant. Collectively, these results reveal a novel role for the newly characterized, Golgi-localized PAQR3 in regulating Gβγ at the non-canonical subcellular location of the Golgi and thus for controlling Golgi-to-PM protein transport via the Gβγ-PKD signaling pathway.
Keywords: Golgi; Heterotrimeric G protein; Membrane transport; Non-canonical signaling; Subcellular localization; Vesicle trafficking.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

