KSHV Latency Locus Cooperates with Myc to Drive Lymphoma in Mice
- PMID: 26327622
- PMCID: PMC4556645
- DOI: 10.1371/journal.ppat.1005135
KSHV Latency Locus Cooperates with Myc to Drive Lymphoma in Mice
Abstract
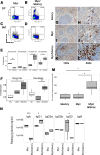
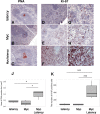
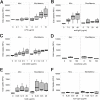
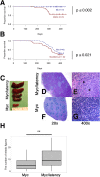
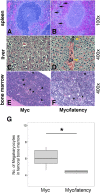
Kaposi sarcoma-associated herpesvirus (KSHV) has been linked to Kaposi sarcoma and B-cell malignancies. Mechanisms of KSHV-induced oncogenesis remain elusive, however, in part due to lack of reliable in vivo models. Recently, we showed that transgenic mice expressing the KSHV latent genes, including all viral microRNAs, developed splenic B cell hyperplasia with 100% penetrance, but only a fraction converted to B cell lymphomas, suggesting that cooperative oncogenic events were missing. Myc was chosen as a possible candidate, because Myc is deregulated in many B cell lymphomas. We crossed KSHV latency locus transgenic (latency) mice to Cα Myc transgenic (Myc) mice. By itself these Myc transgenic mice develop lymphomas only rarely. In the double transgenic mice (Myc/latency) we observed plasmacytosis, severe extramedullary hematopoiesis in spleen and liver, and increased proliferation of splenocytes. Myc/latency mice developed frank lymphoma at a higher rate than single transgenic latency or Myc mice. These data indicate that the KSHV latency locus cooperates with the deregulated Myc pathways to further lymphoma progression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Jaffe ES, Pittaluga S (2011) Aggressive B-cell lymphomas: a review of new and old entities in the WHO classification. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program 2011: 506–514. 10.1182/asheducation-2011.1.506 - DOI - PMC - PubMed
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, et al. (1985) The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318: 533–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

