Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy
- PMID: 26327684
- PMCID: PMC4827375
- DOI: 10.1016/j.bbadis.2015.08.024
Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy
Abstract
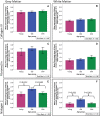
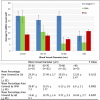
Deposition of amyloid β (Aβ) in the walls of cerebral arteries as cerebral amyloid angiopathy (CAA) suggests an age-related failure of perivascular drainage of soluble Aβ from the brain. As CAA is associated with Alzheimer's disease and with intracerebral haemorrhage, the present study determines the unique sequence of changes that occur as Aβ accumulates in artery walls. Paraffin sections of post-mortem human occipital cortex were immunostained for collagen IV, fibronectin, nidogen 2, Aβ and smooth muscle actin and the immunostaining was analysed using Image J and confocal microscopy. Results showed that nidogen 2 (entactin) increases with age and decreases in CAA. Confocal microscopy revealed stages in the progression of CAA: Aβ initially deposits in basement membranes in the tunica media, replaces first the smooth muscle cells and then the connective tissue elements to leave artery walls completely or focally replaced by Aβ. The pattern of development of CAA in the human brain suggests expansion of Aβ from the basement membranes to progressively replace all tissue elements in the artery wall. Establishing this full picture of the development of CAA is pivotal in understanding the clinical presentation of CAA and for developing therapies to prevent accumulation of Aβ in artery walls. This article is part of a Special Issue entitled: Vascular Contributions to Cognitive Impairment and Dementia edited by M. Paul Murphy, Roderick A. Corriveau and Donna M. Wilcock.
Keywords: Amyloid-β; Basement membranes; Cerebral amyloid angiopathy; Leptomeningeal arteries; Perivascular drainage.
Copyright © 2015 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Thal D.R., von Arnim C., Griffin W.S., Yamaguchi H., Mrak R.E., Attems J. Pathology of clinical and preclinical Alzheimer's disease. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(Suppl 2):S137–S145. - PubMed
-
- Weller R.O., Yow H.Y., Preston S.D., Mazanti I., Nicoll J.A. Cerebrovascular disease is a major factor in the failure of elimination of Abeta from the aging human brain: implications for therapy of Alzheimer's disease. Ann. NY Acad. Sci. 2002;977:162–168. - PubMed
-
- Preston S.D., Steart P.V., Wilkinson A., Nicoll J.A., Weller R.O. Capillary and arterial cerebral amyloid angiopathy in Alzheimer's disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol. Appl. Neurobiol. 2003;29(2):106–117. - PubMed
-
- Attems J., Jellinger K. Neuropathological correlates of cerebral multimorbidity. Curr. Alzheimer Res. 2013;10(6):569–577. - PubMed
-
- Kalaria R.N. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke; J Cereb. Circ. 2012;43(9):2526–2534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

