R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects
- PMID: 26327690
- PMCID: PMC5068814
- DOI: 10.1038/tp.2015.136
R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects
Erratum in
-
Correction: R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects.Transl Psychiatry. 2020 Aug 21;10(1):295. doi: 10.1038/s41398-020-00983-3. Transl Psychiatry. 2020. PMID: 32826855 Free PMC article.
Abstract
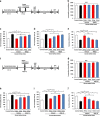
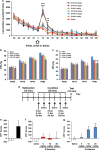
Although the efficacy of racemate ketamine, a rapid onset and sustained antidepressant, for patients with treatment-resistant depression was a serendipitous finding, clinical use of ketamine is limited, due to psychotomimetic side effects and abuse liability. Behavioral and side-effect evaluation tests were applied to compare the two stereoisomers of ketamine. To elucidate their potential therapeutic mechanisms, we examined the effects of these stereoisomers on brain-derived neurotrophic factor (BDNF)-TrkB signaling, and synaptogenesis in selected brain regions. In the social defeat stress and learned helplessness models of depression, R-ketamine showed a greater potency and longer-lasting antidepressant effect than S-ketamine (esketamine). Furthermore, R-ketamine induced a more potent beneficial effect on decreased dendritic spine density, BDNF-TrkB signaling and synaptogenesis in the prefrontal cortex (PFC), CA3 and dentate gyrus (DG) of the hippocampus from depressed mice compared with S-ketamine. However, neither stereoisomer affected these alterations in the nucleus accumbens of depressed mice. In behavioral tests for side effects, S-ketamine, but not R-ketamine, precipitated behavioral abnormalities, such as hyperlocomotion, prepulse inhibition deficits and rewarding effects. In addition, a single dose of S-ketamine, but not R-ketamine, caused a loss of parvalbumin (PV)-positive cells in the prelimbic region of the medial PFC and DG. These findings suggest that, unlike S-ketamine, R-ketamine can elicit a sustained antidepressant effect, mediated by increased BDNF-TrkB signaling and synaptogenesis in the PFC, DG and CA3. R-ketamine appears to be a potent, long-lasting and safe antidepressant, relative to S-ketamine, as R-ketamine appears to be free of psychotomimetic side effects and abuse liability.
Conflict of interest statement
Dr Hashimoto is an inventor on a filed patent application on 'The use of R-ketamine in the treatment of psychiatric diseases' by Chiba University. Dr Hashimoto has served as a scientific consultant to Astellas and Taisho, and he has also received research support from Abbvie, Dainippon Sumitomo, Mochida, Otsuka and Taisho. Dr Shirayama has received research support from Eli Lilly, Eisai, MSD, Otsuka, Pfizer, Taisho, Takeda and Mitsubishi-Tanabe. The remaining authors declare no conflict of interest.
Figures




References
-
- World Health Organization (WHO) Depression. Fact sheet No. 369 2012. Available at http://www.who.int/mediacentre/factsheets/fs369/en/index.html; last accessed on 28 July, 2015.
-
- Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS et al. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry 1999; 7: 125–143. - PubMed
-
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 2009; 61: 105–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

