Liver transplantation for biliary atresia: A single-center study from mainland China
- PMID: 26327772
- PMCID: PMC4548125
- DOI: 10.3748/wjg.v21.i32.9638
Liver transplantation for biliary atresia: A single-center study from mainland China
Abstract
Aim: To summarize our single-center experience with liver transplantation (LT) for biliary atresia (BA).
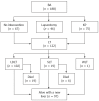
Methods: From October 2006 to December 2012, 188 children with BA were analyzed retrospectively. The stage I group (from October 2006 to December 2010) comprised the first 74 patients, and the stage II group (from January 2011 to December 2012) comprised the remaining 114 patients. Finally, 123 liver transplants were performed in 122 (64.9%) patients, whereas 66 patients did not undergo LT due to denial by their parents or lack of suitable liver grafts. The selection of graft types depended on the patients' clinical status and whether a suitable living donor was available. The characteristics of patients in stages I and II were described, and the surgical outcomes of LT recipients were compared between the two stages. The Kaplan-Meier method was used to estimate the cumulative patient and graft survival rates, and the equality of survival distributions was evaluated using the log-rank test.
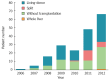
Results: The 188 children consisted of 102 boys and 86 girls. Their ages ranged from 3 to 144 mo with a median of 8 mo. One hundred and fifteen (61.2%) patients were born in rural areas. Comparing stage I and stage II patients, the proportion of patients referred by pediatricians (43.2% vs 71.1%, respectively; P < 0.001) and the proportion of patients who previously received a Kasai procedure (KP) (32.4% vs 44.7%, respectively; P = 0.092) obviously increased, and significantly more parents were willing to treat their children with LT (73% vs 86%, respectively; P = 0.027). Grafts from living donors (102/122, 83.6%) were the most commonly used graft type. Surgical complications (16/25, 64.0%) were the main reason for posttransplant mortality. Among the living donor liver transplantation recipients (n = 102), the incidence of surgical complications was significantly reduced (34.1% vs 15.5%, respectively; P = 0.029) and survival rates of patients and grafts were greatly improved (81.8% vs 89.7%, respectively, at 1 year; 75.0% vs 87.8%, respectively, at 3 years; P = 0.107) from stage I to stage II.
Conclusion: The status of surgical treatments for BA has been changing in mainland China. Favorable midterm outcomes after LT were achieved as centers gained greater technical experience.
Keywords: Biliary atresia; Kasai; Liver transplantation; Living donor; Pediatric; Survival.
Figures


Similar articles
-
Living donor liver transplantation in 43 children with biliary atresia: a single-center experience from the mainland of China.Hepatobiliary Pancreat Dis Int. 2012 Jun;11(3):250-5. doi: 10.1016/s1499-3872(12)60156-8. Hepatobiliary Pancreat Dis Int. 2012. PMID: 22672817
-
Effect of repeat Kasai hepatic portoenterostomy on pediatric live-donor liver graft for biliary atresia.Exp Clin Transplant. 2013 Jun;11(3):259-63. doi: 10.6002/ect.2012.0188. Epub 2013 Mar 26. Exp Clin Transplant. 2013. PMID: 23530849
-
Liver transplantation for biliary atresia: A nationwide investigation from 1996 to 2013 in mainland China.Pediatr Transplant. 2016 Dec;20(8):1051-1059. doi: 10.1111/petr.12750. Epub 2016 Jul 2. Pediatr Transplant. 2016. PMID: 27368158
-
Living donor liver transplantation for biliary atresia.Chang Gung Med J. 2007 Mar-Apr;30(2):103-8. Chang Gung Med J. 2007. PMID: 17595997 Review.
-
Comparison of liver transplantation outcomes in biliary atresia patients with and without prior portoenterostomy: A meta-analysis.Dig Liver Dis. 2016 Apr;48(4):347-52. doi: 10.1016/j.dld.2015.11.021. Epub 2015 Dec 2. Dig Liver Dis. 2016. PMID: 26748427 Review.
Cited by
-
Prognostic Factors Related to In-hospital Death in Children with Biliary Atresia: Analysis of a Nationwide Inpatient Database.J Clin Transl Hepatol. 2023 Apr 28;11(2):416-424. doi: 10.14218/JCTH.2021.00456. Epub 2022 May 17. J Clin Transl Hepatol. 2023. PMID: 36643040 Free PMC article.
-
Pediatric living donor liver transplantation decade progress in Shanghai: Characteristics and risks factors of mortality.World J Gastroenterol. 2020 Mar 28;26(12):1352-1364. doi: 10.3748/wjg.v26.i12.1352. World J Gastroenterol. 2020. PMID: 32256022 Free PMC article.
-
Fat-Soluble Vitamin Deficiency in Pediatric Patients with Biliary Atresia.Gastroenterol Res Pract. 2017;2017:7496860. doi: 10.1155/2017/7496860. Epub 2017 Jun 11. Gastroenterol Res Pract. 2017. PMID: 28690638 Free PMC article.
-
Pediatric and adult liver transplantation in Bahrain: The experiences in a country with no available liver transplant facilities.World J Transplant. 2024 Mar 18;14(1):87752. doi: 10.5500/wjt.v14.i1.87752. World J Transplant. 2024. PMID: 38576753 Free PMC article.
References
-
- Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–1692. - PubMed
-
- McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. 2000;355:25–29. - PubMed
-
- Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard JL, Auvert B. Epidemiology of biliary atresia in France: a national study 1986-96. J Hepatol. 1999;31:1006–1013. - PubMed
-
- Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, Gottrand F, Broué P, Dabadie A, Gauthier F, et al. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58:1209–1217. - PubMed
-
- Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997;99:376–382. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

