Transplantation of bradykinin-preconditioned human endothelial progenitor cells improves cardiac function via enhanced Akt/eNOS phosphorylation and angiogenesis
- PMID: 26328006
- PMCID: PMC4548314
Transplantation of bradykinin-preconditioned human endothelial progenitor cells improves cardiac function via enhanced Akt/eNOS phosphorylation and angiogenesis
Abstract
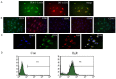
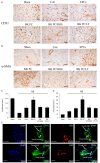
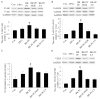
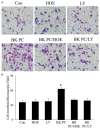
This study determines whether preconditioning (PC) of human endothelial progenitor cells (hEPCs) with bradykinin promotes infarcted myocardium repair via enhanced activation of B2 receptor (B2R)-dependent Akt/eNOS and increased angiogenesis. hEPCs with or without bradykinin preconditioning (BK-PC) were transplanted into a nude mouse model of acute myocardial infarction. Survival of transplanted cells was assessed using DiD-labeled hEPCs. Infarct size, cardiac function, and angiogenesis were measured 10 d after transplantation. Akt, eNOS, and vascular endothelial growth factor (VEGF) expressions in cardiac tissues were detected by western blotting, and NO production was determined using an NO assay kit. The cell migration and tube formation in cultured hEPCs were determined using transwell chamber and matrigel tube formation assays, respectively. The VEGF levels in the cell supernatant were measured using an enzyme-linked immunosorbent assay kit. BK-PC-hEPCs improved cardiac function, decreased infarct size, and promoted neovascularization 10 d following transplantation. PC increased Akt and eNOS phosphorylation, VEGF expression, and NO production in the ischemic myocardium. The effects of BK-PC were abrogated by HOE140 (B2R antagonist) and LY294002 (Akt antagonist). PC increased hEPC migration, tube formation, and VEGF levels in vitro. Activation of B2R-dependent Akt/eNOS phosphorylation by BK-PC promotes hEPC neovascularization and improves cardiac function following transplantation.
Keywords: Bradykinin; endothelial progenitor cells; myocardial infarction; neovascularization; preconditioning.
Figures


References
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. - PubMed
-
- Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387–396. - PubMed
-
- Taljaard M, Ward MR, Kutryk MJ, Courtman DW, Camack NJ, Goodman SG, Parker TG, Dick AJ, Galipeau J, Stewart DJ. Rationale and design of Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction (ENACT-AMI): the first randomized placebo-controlled trial of enhanced progenitor cell therapy for acute myocardial infarction. Am Heart J. 2010;159:354–360. - PubMed
-
- Yu P, Li Q, Liu Y, Zhang J, Seldeen K, Pang M. Pro-angiogenic efficacy of transplanting endothelial progenitor cells for treating hindlimb ischemia in hyperglycemic rabbits. J Diabetes Complications. 2015;29:13–19. - PubMed
LinkOut - more resources
Full Text Sources
