Simplified three-dimensional culture system for long-term expansion of embryonic stem cells
- PMID: 26328022
- PMCID: PMC4550630
- DOI: 10.4252/wjsc.v7.i7.1064
Simplified three-dimensional culture system for long-term expansion of embryonic stem cells
Abstract
Aim: To devise a simplified and efficient method for long-term culture and maintenance of embryonic stem cells requiring less frequent passaging.
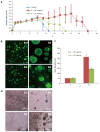
Methods: Mouse embryonic stem cells (ESCs) labeled with enhanced yellow fluorescent protein were cultured in three-dimensional (3-D) self-assembling scaffolds and compared with traditional two-dimentional (2-D) culture techniques requiring mouse embryonic fibroblast feeder layers or leukemia inhibitory factor. 3-D scaffolds encapsulating ESCs were prepared by mixing ESCs with polyethylene glycol tetra-acrylate (PEG-4-Acr) and thiol-functionalized dextran (Dex-SH). Distribution of ESCs in 3-D was monitored by confocal microscopy. Viability and proliferation of encapsulated cells during long-term culture were determined by propidium iodide as well as direct cell counts and PrestoBlue (PB) assays. Genetic expression of pluripotency markers (Oct4, Nanog, Klf4, and Sox2) in ESCs grown under 2-D and 3-D culture conditions was examined by quantitative real-time polymerase chain reaction. Protein expression of selected stemness markers was determined by two different methods, immunofluorescence staining (Oct4 and Nanog) and western blot analysis (Oct4, Nanog, and Klf4). Pluripotency of 3-D scaffold grown ESCs was analyzed by in vivo teratoma assay and in vitro differentiation via embryoid bodies into cells of all three germ layers.
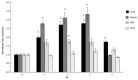
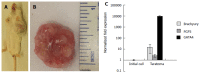
Results: Self-assembling scaffolds encapsulating ESCs for 3-D culture without the loss of cell viability were prepared by mixing PEG-4-Acr and Dex-SH (1:1 v/v) to a final concentration of 5% (w/v). Scaffold integrity was dependent on the degree of thiol substitution of Dex-SH and cell concentration. Scaffolds prepared using Dex-SH with 7.5% and 33% thiol substitution and incubated in culture medium maintained their integrity for 11 and 13 d without cells and 22 ± 5 d and 37 ± 5 d with cells, respectively. ESCs formed compact colonies, which progressively increased in size over time due to cell proliferation as determined by confocal microscopy and PB staining. 3-D scaffold cultured ESCs expressed significantly higher levels (P < 0.01) of Oct4, Nanog, and Kl4, showing a 2.8, 3.0 and 1.8 fold increase, respectively, in comparison to 2-D grown cells. A similar increase in the protein expression levels of Oct4, Nanog, and Klf4 was observed in 3-D grown ESCs. However, when 3-D cultured ESCs were subsequently passaged in 2-D culture conditions, the level of these pluripotent markers was reduced to normal levels. 3-D grown ESCs produced teratomas and yielded cells of all three germ layers, expressing brachyury (mesoderm), NCAM (ectoderm), and GATA4 (endoderm) markers. Furthermore, these cells differentiated into osteogenic, chondrogenic, myogenic, and neural lineages expressing Col1, Col2, Myog, and Nestin, respectively.
Conclusion: This novel 3-D culture system demonstrated long-term maintenance of mouse ESCs without the routine passaging and manipulation necessary for traditional 2-D cell propagation.
Keywords: Embryonic stem cells; Hydrogel; Pluripotency; Self-assembling scaffold; Three-dimensional culture.
Figures




Similar articles
-
Self-Assembling Scaffolds Supported Long-Term Growth of Human Primed Embryonic Stem Cells and Upregulated Core and Naïve Pluripotent Markers.Cells. 2019 Dec 16;8(12):1650. doi: 10.3390/cells8121650. Cells. 2019. PMID: 31888235 Free PMC article.
-
Transcriptomic Analysis of Naïve Human Embryonic Stem Cells Cultured in Three-Dimensional PEG Scaffolds.Biomolecules. 2020 Dec 28;11(1):21. doi: 10.3390/biom11010021. Biomolecules. 2020. PMID: 33379237 Free PMC article.
-
Mechanism of arsenite toxicity in embryonic stem cells.J Appl Toxicol. 2017 Oct;37(10):1151-1161. doi: 10.1002/jat.3469. Epub 2017 Apr 3. J Appl Toxicol. 2017. PMID: 28370166
-
Maintenance of murine embryonic stem cells' self-renewal and pluripotency with increase in proliferation rate by a bovine granulosa cell line-conditioned medium.Stem Cells Dev. 2011 Aug;20(8):1439-49. doi: 10.1089/scd.2010.0336. Epub 2011 Jan 12. Stem Cells Dev. 2011. PMID: 21126164
-
Role of Oct4 in the early embryo development.Cell Regen. 2014 Apr 29;3(1):7. doi: 10.1186/2045-9769-3-7. eCollection 2014. Cell Regen. 2014. PMID: 25408886 Free PMC article. Review.
Cited by
-
Self-Assembling Scaffolds Supported Long-Term Growth of Human Primed Embryonic Stem Cells and Upregulated Core and Naïve Pluripotent Markers.Cells. 2019 Dec 16;8(12):1650. doi: 10.3390/cells8121650. Cells. 2019. PMID: 31888235 Free PMC article.
-
Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications.Stem Cell Rev Rep. 2020 Feb;16(1):3-32. doi: 10.1007/s12015-019-09935-x. Stem Cell Rev Rep. 2020. PMID: 31760627 Free PMC article. Review.
-
Transcriptomic Analysis of Naïve Human Embryonic Stem Cells Cultured in Three-Dimensional PEG Scaffolds.Biomolecules. 2020 Dec 28;11(1):21. doi: 10.3390/biom11010021. Biomolecules. 2020. PMID: 33379237 Free PMC article.
-
Manipulating Living Cells to Construct a 3D Single-Cell Assembly without an Artificial Scaffold.Polymers (Basel). 2017 Jul 30;9(8):319. doi: 10.3390/polym9080319. Polymers (Basel). 2017. PMID: 30970994 Free PMC article.
-
Expression and clinical significance of serum MMP-7 and PTEN levels in patients with acute myeloid leukemia.Oncol Lett. 2018 Mar;15(3):3447-3452. doi: 10.3892/ol.2018.7799. Epub 2018 Jan 15. Oncol Lett. 2018. PMID: 29563992 Free PMC article.
References
-
- Stojkovic M, Lako M, Strachan T, Murdoch A. Derivation, growth and applications of human embryonic stem cells. Reproduction. 2004;128:259–267. - PubMed
-
- Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. - PubMed
-
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

