Syntheses of Sceptrins and Nakamuric Acid and Insights into the Biosyntheses of Pyrrole-Imidazole Dimers
- PMID: 26328059
- PMCID: PMC4551504
- DOI: 10.1039/C5QO00165J
Syntheses of Sceptrins and Nakamuric Acid and Insights into the Biosyntheses of Pyrrole-Imidazole Dimers
Abstract
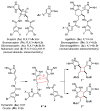
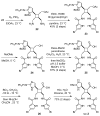
Sceptrins and nakamuric acid are structurally unique antibiotics isolated from marine sponges. Recent studies suggest that the biosynthesis of these dimeric pyrrole-imidazole alkaloids involves a single-electron transfer (SET)-promoted [2+2] cycloaddition to form their cyclobutane core skeletons. We describe herein the biomimetic syntheses of racemic sceptrin and nakamuric acid. We also report the asymmetric syntheses of sceptrin, bromosceptrin, and dibromosceptrin in their natural enantiomeric form. We further provide mechanistic insights into the pathway selectivity of the SET-promoted [2+2] and [4+2] cycloadditions that lead to the divergent formation of the sceptrin and ageliferin core skeletons. Both the [2+2] and [4+2] cycloadditions are stepwise reactions, with the [2+2] pathway kinetically and thermodynamically favored over the [4+2] pathway. For the [2+2] cycloaddition, the dimerization of pyrrole-imidazole monomers is rate-limiting, whereas for the [4+2] cycloaddition, the cyclization is the slowest step.
Figures








Similar articles
-
Total synthesis of dimeric pyrrole-imidazole alkaloids: sceptrin, ageliferin, nagelamide e, oxysceptrin, nakamuric acid, and the axinellamine carbon skeleton.J Am Chem Soc. 2007 Apr 18;129(15):4762-75. doi: 10.1021/ja069035a. Epub 2007 Mar 22. J Am Chem Soc. 2007. PMID: 17375928
-
An approach for the synthesis of nakamuric acid.Tetrahedron. 2015 Jun 3;71(22):3690-3693. doi: 10.1016/j.tet.2014.10.027. Tetrahedron. 2015. PMID: 25983349 Free PMC article.
-
A biomimetic route for construction of the [4+2] and [3+2] core skeletons of dimeric pyrrole-imidazole alkaloids and asymmetric synthesis of ageliferins.J Am Chem Soc. 2012 Nov 14;134(45):18834-42. doi: 10.1021/ja309172t. Epub 2012 Nov 2. J Am Chem Soc. 2012. PMID: 23072663 Free PMC article.
-
Dimeric pyrrole-imidazole alkaloids: sources, structures, bioactivities and biosynthesis.Bioorg Chem. 2023 Apr;133:106332. doi: 10.1016/j.bioorg.2022.106332. Epub 2022 Dec 20. Bioorg Chem. 2023. PMID: 36773454 Review.
-
Dimeric pyrrole-imidazole alkaloids: synthetic approaches and biosynthetic hypotheses.Chem Commun (Camb). 2014 Aug 14;50(63):8628-39. doi: 10.1039/c4cc02290d. Epub 2014 May 15. Chem Commun (Camb). 2014. PMID: 24828265 Free PMC article. Review.
Cited by
-
Marine sponge alkaloids as a source of anti-bacterial adjuvants.Bioorg Med Chem Lett. 2016 Dec 15;26(24):5863-5866. doi: 10.1016/j.bmcl.2016.11.018. Epub 2016 Nov 9. Bioorg Med Chem Lett. 2016. PMID: 27876320 Free PMC article.
-
Rapid access to the core skeleton of the [3 + 2]-type dimeric pyrrole-imidazole alkaloids by triplet ketone-mediated C-H functionalization.Tetrahedron. 2018 Feb 22;74(8):769-772. doi: 10.1016/j.tet.2017.12.027. Epub 2017 Dec 15. Tetrahedron. 2018. PMID: 29622843 Free PMC article.
-
Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria.Nat Prod Rep. 2021 Dec 15;38(12):2214-2235. doi: 10.1039/d0np00078g. Nat Prod Rep. 2021. PMID: 34913053 Free PMC article. Review.
-
Total Synthesis of (±)-Sceptrin.Org Lett. 2020 Sep 4;22(17):6698-6702. doi: 10.1021/acs.orglett.0c01381. Epub 2020 May 7. Org Lett. 2020. PMID: 32379973 Free PMC article.
-
Natural products as inspiration for the development of new synthetic methods.J Chin Chem Soc. 2018 Jan;65(1):43-59. doi: 10.1002/jccs.201700134. Epub 2017 Aug 9. J Chin Chem Soc. 2018. PMID: 29430058 Free PMC article.
References
-
- Walker RP, Faulkner DJ, Engen DV, Clardy J. J Am Chem Soc. 1981;103:6772–6773.
- Assmann M, Köck M. Z Naturforsch, C: Biosci. 2002;57:157–160. - PubMed
-
- Kobayashi J, Tsuda M, Murayama T, Nakamura H, Ohizumi Y, Ishibashi M, Iwamura M, Ohta T, Nozoe S. Tetrahedron. 1990;46:5579–5586.
- Keifer PA, Schwartz RE, Koker MES, Hughes RG, Jr, Rittschof D, Rinehart KL. J Org Chem. 1991;56:2965–2975.
-
- Hoffmann H, Lindel T. Synthesis. 2003;35:1753–1783.
- Jacquot DEN, Lindel T. Curr Org Chem. 2005;9:1551–1565.
- Du H, He Y, Sivappa R, Lovely CJ. Synlett. 2006:965–992.
- Köck M, Grube A, Seiple IB, Baran PS. Angew Chem Int Ed. 2007;46:6586–6594. - PubMed
- Weinreb SM. Nat Prod Rep. 2007;24:931–948. - PubMed
- Arndt HD, Riedrich M. Angew Chem Int Ed. 2008;47:4785–4788. - PubMed
- Heasley B. Eur J Org Chem. 2009:1477–1489.
- Forte B, Malgesini B, Piutti C, Quartieri F, Scolaro A, Papeo G. Mar Drugs. 2009;7:705–753. - PMC - PubMed
- Al-Mourabit A, Zancanella MA, Tilvi S, Romo D. Nat Prod Rep. 2011;28:1229–1260. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
