Preventive nebulization of mucolytic agents and bronchodilating drugs in invasively ventilated intensive care unit patients (NEBULAE): study protocol for a randomized controlled trial
- PMID: 26329352
- PMCID: PMC4557315
- DOI: 10.1186/s13063-015-0865-0
Preventive nebulization of mucolytic agents and bronchodilating drugs in invasively ventilated intensive care unit patients (NEBULAE): study protocol for a randomized controlled trial
Abstract
Background: Preventive nebulization of mucolytic agents and bronchodilating drugs is a strategy aimed at the prevention of sputum plugging, and therefore atelectasis and pneumonia, in intubated and ventilated intensive care unit (ICU) patients. The present trial aims to compare a strategy using the preventive nebulization of acetylcysteine and salbutamol with nebulization on indication in intubated and ventilated ICU patients.
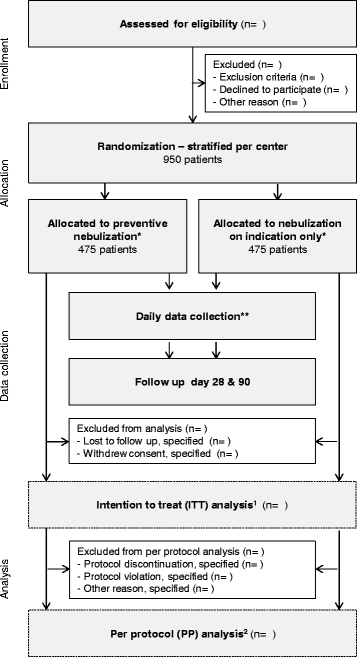
Methods/design: The preventive nebulization of mucolytic agents and bronchodilating drugs in invasively ventilated intensive care unit patients (NEBULAE) trial is a national multicenter open-label, two-armed, randomized controlled non-inferiority trial in the Netherlands. Nine hundred and fifty intubated and ventilated ICU patients with an anticipated duration of invasive ventilation of more than 24 hours will be randomly assigned to receive either a strategy consisting of preventive nebulization of acetylcysteine and salbutamol or a strategy consisting of nebulization of acetylcysteine and/or salbutamol on indication. The primary endpoint is the number of ventilator-free days and surviving on day 28. Secondary endpoints include ICU and hospital length of stay, ICU and hospital mortality, the occurrence of predefined pulmonary complications (acute respiratory distress syndrome, pneumonia, large atelectasis and pneumothorax), and the occurrence of predefined side effects of the intervention. Related healthcare costs will be estimated in a cost-benefit and budget-impact analysis.
Discussion: The NEBULAE trial is the first randomized controlled trial powered to investigate whether preventive nebulization of acetylcysteine and salbutamol shortens the duration of ventilation in critically ill patients.
Trial registration: NCT02159196, registered on 6 June 2014.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

