Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases
- PMID: 26329397
- PMCID: PMC4729787
- DOI: 10.1007/s00520-015-2908-1
Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases
Abstract
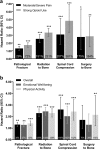
Purpose: Bone metastases secondary to solid tumors increase the risk of skeletal-related events (SREs), including the occurrence of pathological fracture (PF), radiation to bone (RB), surgery to bone (SB), and spinal cord compression (SCC). The aim of this study was to evaluate the impact of SREs on patients' pain, analgesic use, and pain interference with daily functioning.
Methods: Data were combined from patients with solid tumors and bone metastases who received denosumab or zoledronic acid across three identically designed phase 3 trials (N = 5543). Pain severity (worst pain) and pain interference were assessed using the Brief Pain Inventory at baseline and each monthly visit. Analgesic use was quantified using the Analgesic Quantification Algorithm.
Results: The proportion of patients with moderate/severe pain and strong opioid use generally increased in the 6 months preceding an SRE and remained elevated, while they remained relatively consistent over time in patients without an SRE. Regression analysis indicated that all SRE types were significantly associated with an increased risk of progression to moderate/severe pain and strong opioid use. PF, RB, and SCC were associated with significantly greater risk of pain interference overall. Results were similar for pain interference with emotional well-being. All SRE types were associated with significantly greater risk of pain interference with physical function.
Conclusions: SREs are associated with increased pain and analgesic use in patients with bone metastases. Treatments that prevent SREs may decrease pain and the need for opioid analgesics and reduce the impact of pain on daily functioning.
Keywords: Analgesic Quantification Algorithm (AQA); Brief Pain Inventory (BPI); Denosumab; Pain; Skeletal-related events (SREs); Zoledronic acid.
Figures

References
-
- Vadhan-Raj S, von Moos R, Fallowfield LJ, Patrick DL, Goldwasser F, Cleeland CS, Henry DH, Novello S, Hungria V, Qian Y, Feng A, Yeh H, Chung K. Clinical benefit in patients with metastatic bone disease: results of a phase 3 study of denosumab versus zoledronic acid. Ann Oncol. 2012;23(12):3045–3051. doi: 10.1093/annonc/mds175. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

