High LIFr expression stimulates melanoma cell migration and is associated with unfavorable prognosis in melanoma
- PMID: 26329521
- PMCID: PMC4694846
- DOI: 10.18632/oncotarget.4688
High LIFr expression stimulates melanoma cell migration and is associated with unfavorable prognosis in melanoma
Abstract
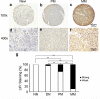
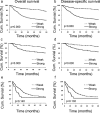
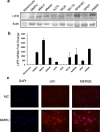
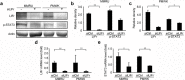
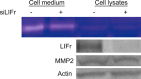
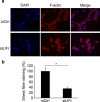
Increased or decreased expression of LIF receptor (LIFr) has been reported in several human cancers, including skin cancer, but its role in melanoma is unknown. In this study, we investigated the expression pattern of LIFr in melanoma and assessed its prognostic value. Using tissue microarrays consisting of 441 melanomas and 96 nevi, we found that no normal nevi showed high LIFr expression. LIFr staining was significantly increased in primary melanoma compared to dysplastic nevi (P = 0.0003) and further increased in metastatic melanoma (P = 0.0000). Kaplan-Meier survival curve and univariate Cox regression analyses showed that increased expression of LIFr was correlated with poorer 5-year patient survival (overall survival, P = 0.0000; disease-specific survival, P = 0.0000). Multivariate Cox regression analyses indicated that increased LIFr expression was an independent prognostic marker for primary melanoma (P = 0.036). LIFr knockdown inhibited melanoma cell migration in wound healing assays and reduced stress fiber formation. LIFr knockdown correlated with STAT3 suppression, but not YAP, suggesting that LIFr activation might stimulate melanoma cell migration through the STAT3 pathway. Our data indicate that strong LIFr expression identifies potentially highly malignant melanocytic lesions at an early stage and LIFr may be a potential target for the development of early intervention therapeutics.
Keywords: LIFr; biomarker; cell migration; melanoma.
Conflict of interest statement
K.M. is Chief Scientific Officer of Replicel Life Sciences Inc. All other authors state no conflict of interest.
Figures


Similar articles
-
Role of Tip60 in human melanoma cell migration, metastasis, and patient survival.J Invest Dermatol. 2012 Nov;132(11):2632-41. doi: 10.1038/jid.2012.193. Epub 2012 Jun 7. J Invest Dermatol. 2012. PMID: 22673729
-
Reduced expression of SRY-box containing gene 17 correlates with an unfavorable melanoma patient survival.Oncol Rep. 2014 Dec;32(6):2571-9. doi: 10.3892/or.2014.3534. Epub 2014 Oct 8. Oncol Rep. 2014. PMID: 25310020
-
Prognostic significance of cytoplasmic p27 expression in human melanoma.Cancer Epidemiol Biomarkers Prev. 2011 Oct;20(10):2212-21. doi: 10.1158/1055-9965.EPI-11-0472. Epub 2011 Aug 9. Cancer Epidemiol Biomarkers Prev. 2011. PMID: 21828232
-
STAT3 targeting by polyphenols: Novel therapeutic strategy for melanoma.Biofactors. 2017 May 6;43(3):347-370. doi: 10.1002/biof.1345. Epub 2016 Nov 29. Biofactors. 2017. PMID: 27896891 Review.
-
TERT and TERT promoter in melanocytic neoplasms: Current concepts in pathogenesis, diagnosis, and prognosis.J Cutan Pathol. 2020 Aug;47(8):710-719. doi: 10.1111/cup.13691. Epub 2020 Apr 3. J Cutan Pathol. 2020. PMID: 32202662 Review.
Cited by
-
Circulating interleukin-8 and osteopontin are promising biomarkers of clinical outcomes in advanced melanoma patients treated with targeted therapy.J Exp Clin Cancer Res. 2024 Aug 15;43(1):226. doi: 10.1186/s13046-024-03151-3. J Exp Clin Cancer Res. 2024. PMID: 39143551 Free PMC article.
-
Leukemia Inhibitory Factor: An Important Cytokine in Pathologies and Cancer.Biomolecules. 2022 Jan 27;12(2):217. doi: 10.3390/biom12020217. Biomolecules. 2022. PMID: 35204717 Free PMC article. Review.
-
Role of the Skin Microenvironment in Melanomagenesis: Epidermal Keratinocytes and Dermal Fibroblasts Promote BRAF Oncogene-Induced Senescence Escape in Melanocytes.Cancers (Basel). 2022 Feb 27;14(5):1233. doi: 10.3390/cancers14051233. Cancers (Basel). 2022. PMID: 35267541 Free PMC article.
-
Cutting to the Chase: How Matrix Metalloproteinase-2 Activity Controls Breast-Cancer-to-Bone Metastasis.Cancers (Basel). 2018 Jun 5;10(6):185. doi: 10.3390/cancers10060185. Cancers (Basel). 2018. PMID: 29874869 Free PMC article. Review.
-
PCBP1 regulates LIFR through FAM3C to maintain breast cancer stem cell self-renewal and invasiveness.Cancer Biol Ther. 2023 Dec 31;24(1):2271638. doi: 10.1080/15384047.2023.2271638. Epub 2023 Nov 6. Cancer Biol Ther. 2023. PMID: 37927213 Free PMC article.
References
-
- Turner RM, et al. Optimizing the Frequency of Follow-Up Visits for Patients Treated for Localized Primary Cutaneous Melanoma. Journal of Clinical Oncology. 2011;29:4641–4646. - PubMed
-
- Geller AC, et al. Reducing mortality in individuals at high risk for advanced melanoma through education and screening. Journal of the American Academy of Dermatology. 2011;65:S87–S94. - PubMed
-
- KellokumpuLehtinen P, et al. Leukemia-inhibitory factor stimulates breast, kidney and prostate cancer cell proliferation by paracrine and autocrine pathways. International Journal of Cancer. 1996;66:515–519. - PubMed
-
- Kamohara H, et al. Leukemia inhibitory factor functions as a growth factor in pancreas carcinoma cells. Involvement of regulation of LIF and its receptor expression. International Journal of Oncology. 2007;30:977–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

