Adenosine deaminase and adenosine kinase expression in human glioma and their correlation with glioma‑associated epilepsy
- PMID: 26329539
- PMCID: PMC4626129
- DOI: 10.3892/mmr.2015.4285
Adenosine deaminase and adenosine kinase expression in human glioma and their correlation with glioma‑associated epilepsy
Abstract
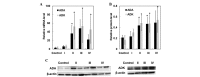
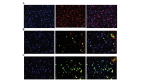
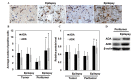
The aim of the present study was to investigate adenosine deaminase (ADA) and adenosine kinase (ADK) expression in human glioma and to explore its correlation with glioma‑associated epilepsy. Tumor tissues (n=45) and peritumoral tissues (n=14) were obtained from glioma patients undergoing surgery. Normal control tissues (n=8) were obtained from brain trauma patients. The disease grade was determined by histological evaluation and the degree of tumor invasion was evaluated using immunofluorescence analyses. mRNA and protein expression of ADA and ADK were evaluated using reverse transcription quantitative polymerase chain reaction or western blot analysis, respectively. Based on histological evaluations, four cases were classified as Grade I gliomas, 18 cases as Grade II, 17 cases as Grade III and six cases were considered Grade IV. Increased ADA and ADK expression was observed in tumor tissues. ADA was predominantly distributed in the cytoplasm of tumor cells, whereas ADK was detected in the cytoplasm as well as in the nuclei. ADA and ADK levels were upregulated in patients with Grade II and Grade III gliomas compared to those in control subjects (p<0.05). In addition, tumor invasion was detected in peritumoral tissues. The number of ADA‑positive or ADK‑positive cells in tumor tissues was similar between glioma patients with and without epilepsy (p>0.05). However, ADA and ADK expression was upregulated in peritumoral tissues derived from patients with epilepsy compared to that in glioma patients without epilepsy. The results of the present study suggested that ADA and ADK are involved in glioma progression, and that increased ADA and ADK levels in peritumoral tissues may be associated with epilepsy in glioma patients.
Figures


References
-
- Pace A, Bove L, Innocenti P, Pietrangeli A, Carapella CM, Oppido P, Raus L, Occhipinti E, Jandolo B. Epilepsy and gliomas: Incidence and treatment in 119 patients. J Exp Clin Cancer Res. 1998;17:479–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

