Is Blood Eosinophil Count a Predictor of Response to Bronchodilators in Chronic Obstructive Pulmonary Disease? Results from Post Hoc Subgroup Analyses
- PMID: 26329916
- PMCID: PMC4579251
- DOI: 10.1007/s40261-015-0322-6
Is Blood Eosinophil Count a Predictor of Response to Bronchodilators in Chronic Obstructive Pulmonary Disease? Results from Post Hoc Subgroup Analyses
Abstract
Background: Chronic obstructive pulmonary disease (COPD) patients with blood eosinophil (EOS) count ≥ 2% benefit from exacerbation reductions with inhaled corticosteroids (ICSs). We conducted post hoc analyses to determine if EOS count ≥ 2% is a marker for greater responsiveness to the bronchodilators umeclidinium (UMEC; long-acting muscarinic antagonist), vilanterol (VI; long-acting β2-agonist) or UMEC/VI combination.
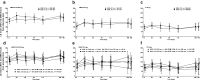
Methods: Effects of once-daily UMEC/VI 62.5/25, UMEC 62.5 and VI 25 µg versus placebo on trough forced expiratory volume in one second (FEV1), Transition Dyspnoea Index (TDI), St George's Respiratory Questionnaire (SGRQ) scores and adverse event (AE) incidences in four completed, 6-month studies were assessed by EOS subgroup. Trough FEV1 was also evaluated by ICS use and EOS subgroup. Analyses were performed using a repeated measures model.
Results: At baseline, 2437 of 4647 (52%) patients had EOS count ≥ 2%. Overall, ≈ 50% of patients used ICSs. At day 169, no notable variations were observed in trough FEV1 least squares mean differences between EOS subgroups versus placebo for UMEC/VI, UMEC and VI; results according to ICS use were similar. No differences were reported between EOS subgroups in TDI and SGRQ scores on day 168, or for incidences of AEs, serious AEs and AEs leading to withdrawal.
Conclusions: Response to UMEC/VI, UMEC and VI in terms of trough FEV1, dyspnoea and health-related quality of life was similar for COPD patients with baseline EOS counts ≥ 2 or <2%. EOS count did not appear to predict bronchodilator response in either ICS users or non-users.
Figures
References
-
- Price D, Rigazio A, Postma D, Papi A, Guy B, Agusti A, et al. Blood eosinophilia and the number of exacerbations in COPD patients [abstract] Eur Respir J. 2014;44(Suppl. 58):4416.
-
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. - DOI - PMC - PubMed
-
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

