Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity
- PMID: 26330164
- PMCID: PMC4624228
- DOI: 10.1158/0008-5472.CAN-15-0139
Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity
Abstract
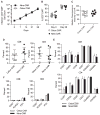
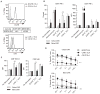
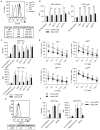
Many tumors overexpress tumor-associated antigens relative to normal tissue, such as EGFR. This limits targeting by human T cells modified to express chimeric antigen receptors (CAR) due to potential for deleterious recognition of normal cells. We sought to generate CAR(+) T cells capable of distinguishing malignant from normal cells based on the disparate density of EGFR expression by generating two CARs from monoclonal antibodies that differ in affinity. T cells with low-affinity nimotuzumab-CAR selectively targeted cells overexpressing EGFR, but exhibited diminished effector function as the density of EGFR decreased. In contrast, the activation of T cells bearing high-affinity cetuximab-CAR was not affected by the density of EGFR. In summary, we describe the generation of CARs able to tune T-cell activity to the level of EGFR expression in which a CAR with reduced affinity enabled T cells to distinguish malignant from nonmalignant cells.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures
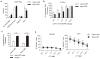


References
-
- Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. Journal of neuro-oncology. 2012;107(2):359–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA141303/CA/NCI NIH HHS/United States
- CA124782/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA124782/CA/NCI NIH HHS/United States
- CA16672/CA/NCI NIH HHS/United States
- CA120956/CA/NCI NIH HHS/United States
- R01 CA163587/CA/NCI NIH HHS/United States
- TL1TR000369/TR/NCATS NIH HHS/United States
- R01 CA174385/CA/NCI NIH HHS/United States
- P50 CA136411/CA/NCI NIH HHS/United States
- P01 CA148600/CA/NCI NIH HHS/United States
- CA141303/CA/NCI NIH HHS/United States
- R01 CA120956/CA/NCI NIH HHS/United States
- TL1 TR000369/TR/NCATS NIH HHS/United States
- P01 CA030206/CA/NCI NIH HHS/United States
- CA148600/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

